
Hydrogen-Bonding Research in Photochemistry, Photobiology, and Optoelectronic Materials
- 456 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Hydrogen-Bonding Research in Photochemistry, Photobiology, and Optoelectronic Materials
About this book
As one of the typical intermolecular interactions, hydrogen-bonding plays a significant role in molecular structure and function. When the hydrogen bond research system is connected with the photon, the hydrogen-bonding effect turns to an excited-state one influencing photochemistry, photobiology, and photophysics. Thus, the hydrogen bond in an excited state is a key topic for understanding the excited-state properties, especially for optoelectronic or luminescent materials.
The approaches presented in this book include quantum chemical calculation, molecular dynamics simulation and ultrafast spectroscopy, which are strong tools to investigate the hydrogen bond. Unlike other existing titles, this book combines theoretical calculations and experiments to explore the nature of excited-state hydrogen bonds. By using these methods, more details and faster processes involved in excited-state dynamics of hydrogen bond are explored.
This highly interdisciplinary book provides an overview of leading hydrogen bond research. It is essential reading for faculties and students in researching photochemistry, photobiology and photophysics, as well as novel optoelectronic materials, fluorescence probes and photocatalysts. It will also guide research beginners to getting a quick start within this field.
Contents:
- Effect of Solute-Solvent Hydrogen-Bonding on Spectral and Photophysical Properties of Aromatic Probes (Ewa Krystkowiak)
- Intramolecular and Intra-Assembly Triplet Energy Transfer (Kepeng Chen and Jianzhang Zhao)
- Elucidating Excited-State Hydrogen-Bonding Dynamics and Proton Transfer Inside Fluorescent Protein Calcium Biosensors (Longteng Tang and Chong Fang)
- Proton-Coupled Electron Transfer (PCET) Mechanism of Identify Hydrogen-Atom Exchange Reaction — Two Transition States in the Vicinity of Conical Intersection (Shmuel Zilberg)
- Probing Dynamics of Nonfluorescent Excited-State Intramolecular Proton Transfer (Ying Shi and Hang Yin)
- Excited-State Dynamics of Intermolecular Dihydrogen Bond in Different Systems (Ningning Wei and Guangjiu Zhao)
- Computational Studies on the Excited-State Intramolecular Proton Transfer in Five-Membered-Ring Hydrogen-Bonded Systems (Renuka Pradhan and Upakarasamy Lourderaj)
- Theoretical Investigation of Excited-State Intramolecular Proton Transfer Mechanism of Flavonoid Derivatives (Zhe Tang, Yi Wang, and Ningjiu Zhao)
- Photophysical Processes of Organic Chromophores with Intramolecular and Intermolecular Hydrogen Bonds (Shuo Chai and Shu-Lin Cong)
- Stronger Hydrogen Bonds of Less Stable Tautomers in the Ground State and Reversed Stability in the First Excited State: The Role of Electronic Excited States in Hydrogen-Bonding (Shmuel Zilberg)
- Effects of Hydrogen Bond and π–π Stacking on the Luminescence of Metal-Organic Frameworks (Lei Liu and Dan Zhao)
- Excited-State Hydrogen-Bonding Dynamics of Polyaniline Complex (Yahong Zhang, Dan Zhao, and Mingxing Zhang)
- Femtosecond Stimulated Raman Spectrocopy (FSRS) Investigation of Excited State Hydrogen-Bonding Dynamics and Photoacidity in Solution (Yanli Wang and Chong Fang)
- TD/DFT Study of Hydrogen-Bonding-Concerned System in the Excited State (Dandan Wang and Peng Li)
- Excited-State Hydrogen-Bonding Dynamics and Coupled Proton Transfer for Luminous Molecules (Mingzhen Zhang, Baiping Ren, and Yi Wang)
- Exploring and Elaborating Excited-State Intramolecular Double-Proton Transfer Mechanisms (Jinfeng Zhao and Yujun Zheng)
Readership: Hydrogen-bonding researchers, including faculties, post-doctors, and graduate students. It will serve as a good reference book for all researchers to gain knowledge about the excited-state hydrogen-bonding information. Hydrogen-Bonding;Photochemistry;Photobiology;Optoelectronic Materials00
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
Chapter 1
Effect of Solute–Solvent Hydrogen-Bonding on Spectral and Photophysical Properties of Aromatic Probes
Umultowska 89b, Poznań, Poland
[email protected]
Introduction
Solvent selection — important study point
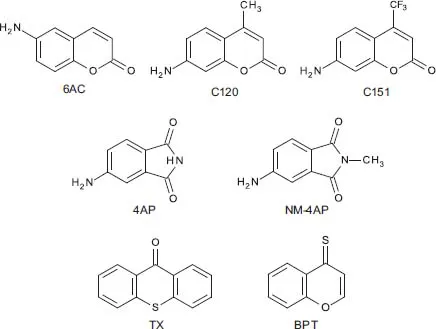
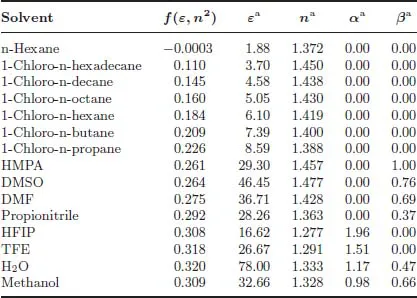
aFrom Ref. [33].
Table of contents
- Cover
- Halftitle
- Series Editors
- Title
- Copyright
- Preface
- About the Editors
- Contents
- 1. Effect of Solute–Solvent Hydrogen-Bonding on Spectral and Photophysical Properties of Aromatic Probes
- 2. Intramolecular and Intra-assembly Triplet Energy Transfer
- 3. Elucidating Excited-State Hydrogen-Bonding Dynamics and Proton Transfer inside Fluorescent Protein Calcium Biosensors
- 4. Proton-Coupled Electron Transfer (PCET) Mechanism to Identify Hydrogen-Atom Exchange Reaction — Two Transition States in the Vicinity of Conical Intersection
- 5. Probing Dynamics of Nonfluorescent Excited-State Intramolecular Proton Transfer
- 6. Excited-State Dynamics of Intermolecular Dihydrogen Bond in Different Systems
- 7. Computational Studies on the Excited-State Intramolecular Proton Transfer in Five-Membered-Ring Hydrogen-Bonded Systems
- 8. Theoretical Investigation of Excited-State Intramolecular Proton Transfer Mechanism of Flavonoid Derivatives
- 9. Photophysical Processes of Organic Chromophores with Intramolecular and Intermolecular Hydrogen Bonds
- 10. Stronger Hydrogen Bonds of Less Stable Tautomers in the Ground State and Reversed Stability in the First Excited State: The Role of Electronic Excited States in Hydrogen-Bonding
- 11. Effects of Hydrogen Bond and π–π Stacking on the Luminescence of Metal-Organic Frameworks
- 12. Excited-State Hydrogen-Bonding Dynamics of Polyaniline Complex
- 13. Femtosecond Stimulated Raman Spectroscopy (FSRS) Investigation of Excited-State Hydrogen-Bonding Dynamics and Photoacidity in Solution
- 14. TD/DFT Study of Hydrogen-Bonding-Concerned System in the Excited State
- 15. Excited-State Hydrogen-Bonding Dynamics and Coupled Proton Transfer for Luminous Molecules
- 16. Exploring and Elaborating Excited-State Intramolecular Double-Proton Transfer Mechanisms
- Index