
- 304 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
About this book
This richly illustrated book written by Professor Kai Peter Birke and several co-authors addresses both scientific and engineering aspects of modern batteries in a unique way. Emphasizing the engineering part of batteries, the book acts as a compass towards next generation batteries for automotive and stationary applications. The book provides distinguished answers to still open questions on how future batteries look like.
Modern Battery Engineering explains why and how batteries have to be designed for successful commercialization in e-mobility and stationary applications. The book will help readers understand the principle issues of battery designs, paving the way for engineers to avoid wrong paths and settle on appropriate cell technologies for next generation batteries. This book is ideal for training courses for readers interested in the field of modern batteries.
Contents:
- Fundamental Aspects of Achievable Energy Densities in Electrochemical Cells (Kai Peter Birke and Desirée Nadine Schweitzer)
- Lithium-ion Cells: Discussion of Different Cell Housings (Kai Peter Birke and Shkendije Demolli)
- Integral Battery Architecture with Cylindrical Cells as Structural Elements (Christoph Bolsinger, Marcel Berner and Kai Peter Birke)
- Parallel Connection of Lithium-ion Cells — Purpose, Tasks and Challenges (Alexander Fill)
- Fundamental Aspects of Reconfigurable Batteries: Efficiency Enhancement and Lifetime Extension (Nejmeddine Bouchhima, Matthias Gossen and Kai Peter Birke)
- Volume Strain in Lithium Batteries (Jan Patrick Singer and Kai Peter Birke)
- Every Day a New Battery: Aging Dependence of Internal States in Lithium-ion Cells (Severin Hahn and Kai Peter Birke)
- Thermal Propagation (Sascha Koch)
- Potential of Capacitive Effects in Lithium-ion Cells (Alexander Uwe Schmid and Kai Peter Birke)
- Battery Recycling: Focus on Li-ion Batteries (Daniel Horn, Jörg Zimmermann, Andrea Gassmann, Rudolf Stauber and Oliver Gutfleisch)
- Power-to-X Conversion Technologies (Friedrich-Wilhelm Speckmann and Kai Peter Birke)
Readership: Engineers and professionals dealing with or specialising in cell technologies and their R&D. Also provides an advanced training course for anyone interested in the field of modern batteries.Batteries;Battery Engineering;Electrical Energy Storage Systems;Battery Management Systems;Lithium-Ion;Post Lithium-Ion;Metal-Air Batteries;Battery Modeling;Aging Effects in Batteries;Thermal Management;Thermal Propagation;Recycling;Pseudo-Capacitors Electrolysis0 Key Features:
- Unique treatment of selected aspects of battery engineering, new edition/material, joint comprehensive knowledge of several authors doing advanced research and engineering on batteries
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Chapter 1
Fundamental Aspects of Achievable Energy Densities in Electrochemical Cells

AM (AM = Active material) can be easily derived from the periodic table [2].
m in the appropriate unit 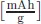
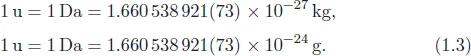
A, which is simply the number of particles in one mole.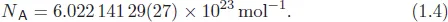
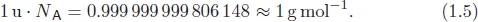
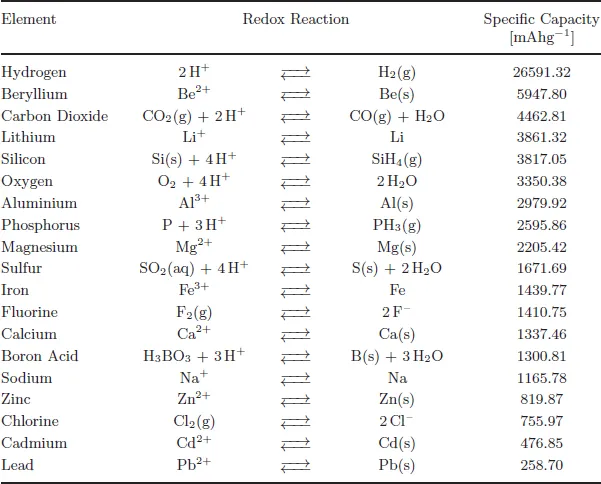
m of an element or compound will be able to provide a deeper insight than its specific capacity? The gravimetric energy density of an element shows how much energy per kilogram or gram can be stored, while its volumetric energy density shows how much energy per litre can be stored. In this regard these are the most important values to differentiate potential candidates for high-energy cells. To calculate the gravimetric energy density Em of a single element or compound, the specific capacity Cm is multiplied with the electrochemical series voltage U0, as shown in Eq. (1.6).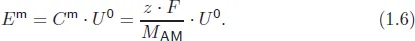
Table of contents
- Cover Page
- Title Page
- Copyright Page
- Contents
- Preface
- About the Editor
- About the Authors
- 1. Fundamental Aspects of Achievable Energy Densities in Electrochemical Cells
- 2. Lithium-ion Cells: Discussion of Different Cell Housings
- 3. Integral Battery Architecture with Cylindrical Cells as Structural Elements
- 4. Parallel Connection of Lithium-ion Cells — Purpose, Tasks and Challenges
- 5. Fundamental Aspects of Reconfigurable Batteries: Efficiency Enhancement and Lifetime Extension
- 6. Volume Strain in Lithium Batteries
- 7. Every Day a New Battery: Aging Dependence of Internal States in Lithium-ion Cells
- 8. Thermal Propagation
- 9. Potential of Capacitive Effects in Lithium-ion Cells
- 10. Battery Recycling: Focus on Li-ion Batteries
- 11. Power-to-X Conversion Technologies
- Epilogue
- Acknowledgments
- Index
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app