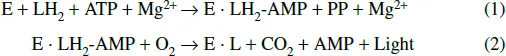![]()
CHAPTER 1
The Fireflies and Luminous Insects
Osamu Shimomuraa, Yuichi Obab, Alexey A. Kotlobayc and Aleksandra S. Tsarkovac
Since ancient times, the light emitted by fireflies and glow-worms has attracted the curiosity of people. Descriptions of the phenomena are frequently found in old poems, songs, and the folklore of many countries. Old scientific studies on these phenomena are also numerous, particularly after the 17th century. However, the chemical study of bioluminescent insects began only in the early 20th century.
Although the insects (the subphylum Hexapoda in the phylum Arthropoda) contain bioluminescent organisms in three orders: Collembola, Coleoptera, and Diptera, biochemical studies have been carried out only with several types of organisms of the last two orders, Coleoptera and Diptera. In these orders, the adults have two pairs of wings: In Coleoptera, the front wings are modified as elytra (a heavy protective cover), and in Diptera, the hind wings are reduced to knobs. The order Coleoptera includes Lampyridae (fireflies), Phengodidae (railroad worms), and Elateridae (click beetles such as Pyrophorus), and all the luminous species in this order utilize firefly luciferin in their light emission. The order Diptera contains the glow-worms Arachnocampa, Keroplatus, and Orfelia, and their bioluminescence systems are clearly different from that of Coleoptera.
According to Harvey (1952), there are considerable differences in morphology between the male and the female among various genera of fireflies (lampyrids). In many of them, both the male and the female are winged and can fly, but the male usually has a larger light organ than the female. They use light signals to find each other. In some cases, females are luminous but males are not, and some species do not emit light at all. In Lucidota and Pyropyga, the larvae are luminous but the adult fireflies are not. The eggs of the fireflies are generally luminous — this was discovered as early as 1643 by Thomas Bartholin. The functions of the light emission of the fireflies and the behaviors of synchronous flashing have been reviewed by John B. Buck (Buck and Buck, 1976; Buck, 1978).
In northern and central Europe, the most common lampyrid is the glow-worm Lampyris. The female of this genus is wingless and has a bright light organ, which attracts the flying male that has a less bright light organ. In Italy and southern Europe, the most common fireflies belong to the genus Luciola where both the male and the female are winged. In North America, the genera Photinus and Photuris are most common. Some of the Photinus females have partially developed wings and do not fly. Photuris is carnivorous and eats fireflies of other species.
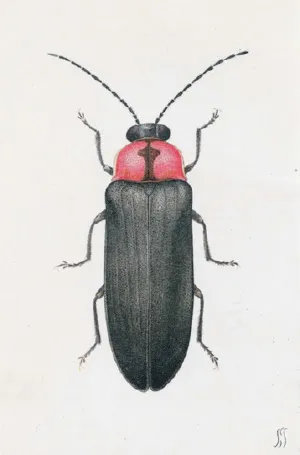
Lampyrids are abundant in Japan. Ohba (1997, 2004) lists nine genera of lampyrids containing more than 50 species. The most common of them are Luciola cruciata (Fig. 1.1) and Luciola lateralis. These two species live in water during their larval stage (1 or 2 years), making a distinct difference from all other species of fireflies; the larvae are luminescent. These lampyrids belong to one of the two kinds of luminous organisms that inhabit freshwater; the other is the New Zealand freshwater limpet Latia.
The fireflies, railroad worms, and click beetles use the same luciferin in their luminescence reactions. Recent studies on the railroad worms and the click beetles have greatly contributed to the biochemical understanding of the firefly bioluminescence (see Section 1.2). Concerning luminous Diptera, significant progress has been made only recently.
Fig. 1.1The firefly L. cruciata (male) drawn in the book by Sakyo Kanda (1874–1939), a pioneer of the study of bioluminescence in Japan. (Reproduced from Kanda, 1935).
1.1The Fireflies
1.1.1An overview of the firefly luminescence reaction
The luciferin–luciferase reaction of fireflies was first demonstrated by Harvey (1917), although the light observed was weak and short lasting. Thirty years after Harvey’s discovery, McElroy (1947) made a crucial breakthrough in the study of firefly bioluminescence. He found that the light-emitting reaction requires ATP as a co-factor. The addition of ATP to the mixtures of luciferin and luciferase resulted in a bright, long-lasting luminescence. It was not a simple experiment because ATP was not commercially available at the time. McElroy prepared ATP from rabbit muscles. The discovery of the cofactor ATP was extremely important indeed, and it cleared the way for the spectacular, rapid progress in the chemical study of firefly bioluminescence.
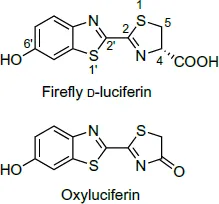
Fig. 1.2Structures of firefly luciferin and oxyluciferin.
In 1949, McElroy and Strehler found that the luminescence reaction requires Mg2+ in addition to luciferin, luciferase, and ATP. The luciferase was partially purified and various characteristics of the luminescence reaction were investigated by McElroy and Hastings (1955). The luciferase was crystallized by Green and McElroy (1956). The luciferin was purified and crystallized by Bitler and McElroy (1957), which led to the determination of its structure and chemical synthesis (White et al., 1961, 1963). The active luciferin was found to be in D-form (Fig. 1.2); the L-form is practically inactive. According to a study by Lembert (1996), L-luciferin is a competitive inhibitor of firefly luciferase, although it can produce some light. The mechanism of the chemiluminescence of luciferin that involves a dioxetanone intermediate was first proposed by Hopkins et al. (1967) and McCapra et al. (1968). The dioxetanone mechanism in the luciferasecatalyzed luminescence reaction was experimentally confirmed by 18O-labeling studies (Shimomura et al., 1977; Wannlund et al., 1978).
The following schemes represent the overall reaction of firefly bioluminescence (McElroy and DeLuca, 1978), where E is luciferase, LH2 is D-luciferin, PP is pyrophosphate, AMP is adenosine phosphate, LH2-AMP is D-luciferyl adenylate (an anhydride formed between the carboxyl group of luciferin and the phosphate group of AMP), and L is oxyluciferin.
In the first step, luciferin is converted into luciferyl adenylate by ATP in the presence of Mg2+. In the second step, luciferyl adenylate is oxidized by molecular oxygen resulting in the emission of yellow-green light, of which the mechanism is discussed in Sections 1.1.6 and 1.1.7. Both steps, (1) and (2), are catalyzed by luciferase. The reaction in the first step is slower than that in the second step, thus the first step is the rate-limiting step.
1.1.2Firefly luciferin and oxyluciferin
Extraction and purification of luciferin. In the work of purifying and crystallizing firefly luciferin (Bitler and McElroy, 1957), McElroy used a unique method to gather the large quantity of fireflies needed for their research. In the now a legendary story, they advertised to buy fireflies at one cent per specimen. Children and youths in the neighborhood responded enthusiastically, collecting a huge number of the bugs for them. In this way, they easily obtained a sufficient quantity of the firefly Photinus pyralis for their research.
The live fireflies are dried over calcium chloride in a vacuum desiccator, and the lanterns are then separated by hand. An acetone powder prepared from the dried lanterns is extracted with boiling water. The cooled aqueous extract is extracted with ethyl acetate at pH 3.0, and the ethyl acetate layer is concentrated under reduced pressure. The concentrated luciferin is adsorbed on a column of Celite-Fuller’s earth mixture. The column is washed with water-saturated ethyl acetate, and eluted with alkaline water at pH 8.0–8.5. The aqueous eluate of luciferin is adjusted to pH 3.0 with HCl and luciferin is re-extracted with ethyl acetate. Luciferin is further purified by partition chromatography on a column of Celite using a mixture of butanol–chloroform–water (135:15:50) as the elution solvent, monitoring the absorbance values at 327 and 276 nm; the former value parallels with the concentration of luciferin, whereas the latter indicates the protein concentration. The purity is judged by the absorbance ratio of 327/276. For crystallization, an aqueous solution of luciferin is extracted with ethyl acetate at pH 3.0. The ethyl acetate layer is washed with water, then evaporated to dryness. The residue of free (acidic) form of luciferin is dissolved in about 3.5 mL of acetone, and 1.5 mL of water is added. The acetone is slowly evaporated by bubbling a stream of nitrogen gas until crystals are formed. From 70 g of acetone powder (about 15,000 fireflies), Bitler and McElroy obtained 9 mg of crystalline luciferin.
In 1968, Kishi et al. reported another method for purifying luciferin from the Japanese firefly L. cruciata. In this method, the first ethyl acetate extract from Bitler and McElroy’s process was chromatographed on a column of cellulose powder using ethyl acetate–ethanol–water (5:2:3) as the eluting solvent, followed by chromatography on a column of DEAE-cellulose (elution with a gradient of NaCl concentration). They obtained 5.5 mg of crystalline luciferin from 233 g of the acetone powder from the abdomens (from 12,000 fireflies). Both HPLC with fluorescence detection and luciferin–luciferase reaction analyses showed that male adult L. cruciata contains approximately 3.6 nmol/insect of luciferin (Oba et al., 2008).
Properties of luciferin. The crystals are microscopic needles, which melt with decomposition at 205–210°C (Bitler and McElroy, 1957). It is a quite stable luciferin compared with some other luciferins, such as Cypridina luciferin and the luciferins of krill and dinoflagellates. It is not significantly affected by 10 mM H2SO4 and 10 mM NaOH at room temperature in air. The absorption spectral data of luciferin are shown in Fig. 1.3 (McElroy and Seliger, 1961). The molar absorption coefficient of the 328 nm peak in acidic solutions and that of the 384 nm peak in basic solutions are both 18,200 (Morton et al., 1969). Luciferin is fluorescent showing an emission maximum at 537 nm in both acidic and basic conditions, although the intensity of the fluorescence is lower in acidic solution than in basic solution (fluorescence quantum yields 0.62 in basic condition and 0.25 in acidic condition; Morton et al., 1969). The chemical synthesis of luciferin was accomplished by White et al. (1961, 1963); certain details and the improvements of...