
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Computational Pharmaceutical Solid State Chemistry
About this book
This book is the first to combine computational material science and modeling of molecular solid states for pharmaceutical industry applications. • Provides descriptive and applied state-of-the-art computational approaches and workflows to guide pharmaceutical solid state chemistry experiments and to support/troubleshoot API solid state selection
• Includes real industrial case examples related to application of modeling methods in problem solving
• Useful as a supplementary reference/text for undergraduate, graduate and postgraduate students in computational chemistry, pharmaceutical and biotech sciences, and materials science
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1
COMPUTATIONAL PHARMACEUTICAL SOLID-STATE CHEMISTRY: AN INTRODUCTION
Yuriy A. Abramov
Pfizer Worldwide Research & Development, Groton, CT, USA
1.1 INTRODUCTION
Traditionally, pharmaceutical industry is focusing on discovery and manufacturing of small-molecule drug compounds. Pharmaceutical industry workflow is characterized by two somewhat overlapping stages—Drug Discovery and Drug Development. At the first stage, a new chemical entity (drug candidate molecule for clinical development) is being discovered and tested on animals. At the end of this stage it is important to make sure that the selected molecule passes preclinical testing such as in vivo biological activity in animal models, in vitro metabolism, pharmacokinetic profiling in animals, and animal toxicology studies. The drug candidate progresses into an early development stage to pass proof of concept (POC), which refers to early clinical studies on human divided into Phase I and Phase IIa. At this step the candidate molecule becomes an active pharmaceutical ingredient (API) of drug product and is typically formulated in a solid form. The subsequent Drug Development process is focused on drug product and process development to ensure reliable performance, manufacturing, and storage.
Along the pharmaceutical industry workflow path, a drug substance undergoes a significant physical transformation (Fig. 1.1). It starts in early Drug Discovery as a single molecule (ligand) binding to a receptor in order to activate or inhibit the receptor’s associated biochemical pathway. Then the drug molecule becomes a biologically active component of a typically solid-state (e.g., crystalline or amorphous) formulation in early Drug Development. Finally, the drug molecule acts as an API of the solid particles of the drug product at the later stages of Drug Development. This transformational pathway reflects the complex nature of the drug design workflow and dictates a diversity of experimental and especially computational methods, which are applied to support Drug Discovery and Drug Development.
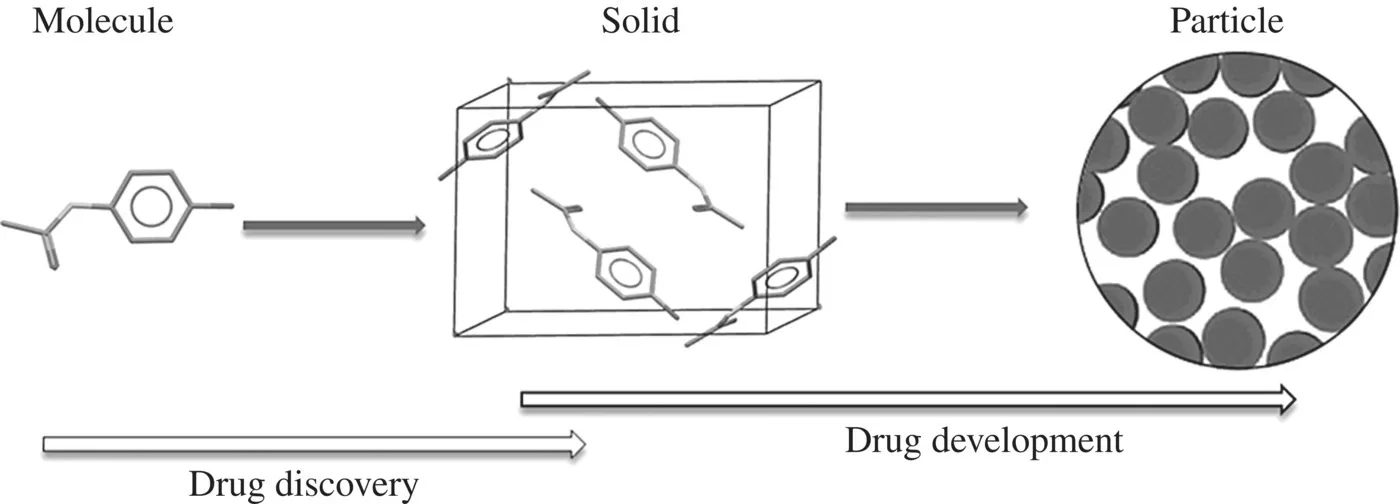
Figure 1.1 Physical transformation of a drug substance along the pharmaceutical industry workflow.
The pharmaceutical industry as a whole has faced many challenges in recent years in addition to patent expirations of blockbuster drugs. In particular, the Drug Development branch faces challenges of accelerated development under a high regulatory pressure. An ability to rationalize and guide Drug Development process has become crucial [1]. Computational chemistry methods have become deeply integrated into Drug Discovery over the past 30 years [2, 3]. However, the computational support of Drug Development has emerged only in recent years and is now tasked with the whole spectrum of Drug Development fields including drug formulation and product design, process chemistry, chemical engineering and analytical research and development. This chapter provides a high-level overview of pharmaceutical solid-state landscape and introduces a field of computational modeling in Drug Development, hereinafter called computational pharmaceutical solid-state chemistry (CPSSC).
1.2 PHARMACEUTICAL SOLID-STATE LANDSCAPE
1.2.1 Some Definitions
Approximately 70% of the drug products marketed worldwide are formulated in oral solid dosage forms [4]. The pharmaceutical solid state may be characterized by molecular arrangement displaying long-range order in all directions (crystalline), long-range order in one or two directions (liquid crystals), or only close-range order (amorphous). An overall pharmaceutical solid-state landscape is presented in Figure 1.2. The advantage of formulation of drug substances in crystalline form is dictated by more desirable manufacturing properties: superior stability, purity, and manufacturability relative to amorphous and liquid form formulations. All solid drugs can be subclassified as single- (anhydrous) and multicomponent compounds. Multicomponent substances can be crystalline solvates (including solid hydrates) [5, 6], cocrystals (or co-crystals) [7], and salts [8]. Solid solvates (also named pseudopolymorphs or solvatomorphs) represent crystal structures in which solvent molecules are integrated into the crystal lattice. Solid hydrates are the most common pharmaceutical pseudopolymorphs. Pharmaceutical cocrystals are defined as stoichiometric multicomponent crystals formed by an API (or an intermediate compound) with at least one cocrystal former (coformer), which is solid at ambient temperature. Within the family of solvates, hydrates, and cocrystals, the components are neutral. Pharmaceutical salts are multicomponent materials in which components are ionized via proton transfer and are involved in ionic interactions with each other.
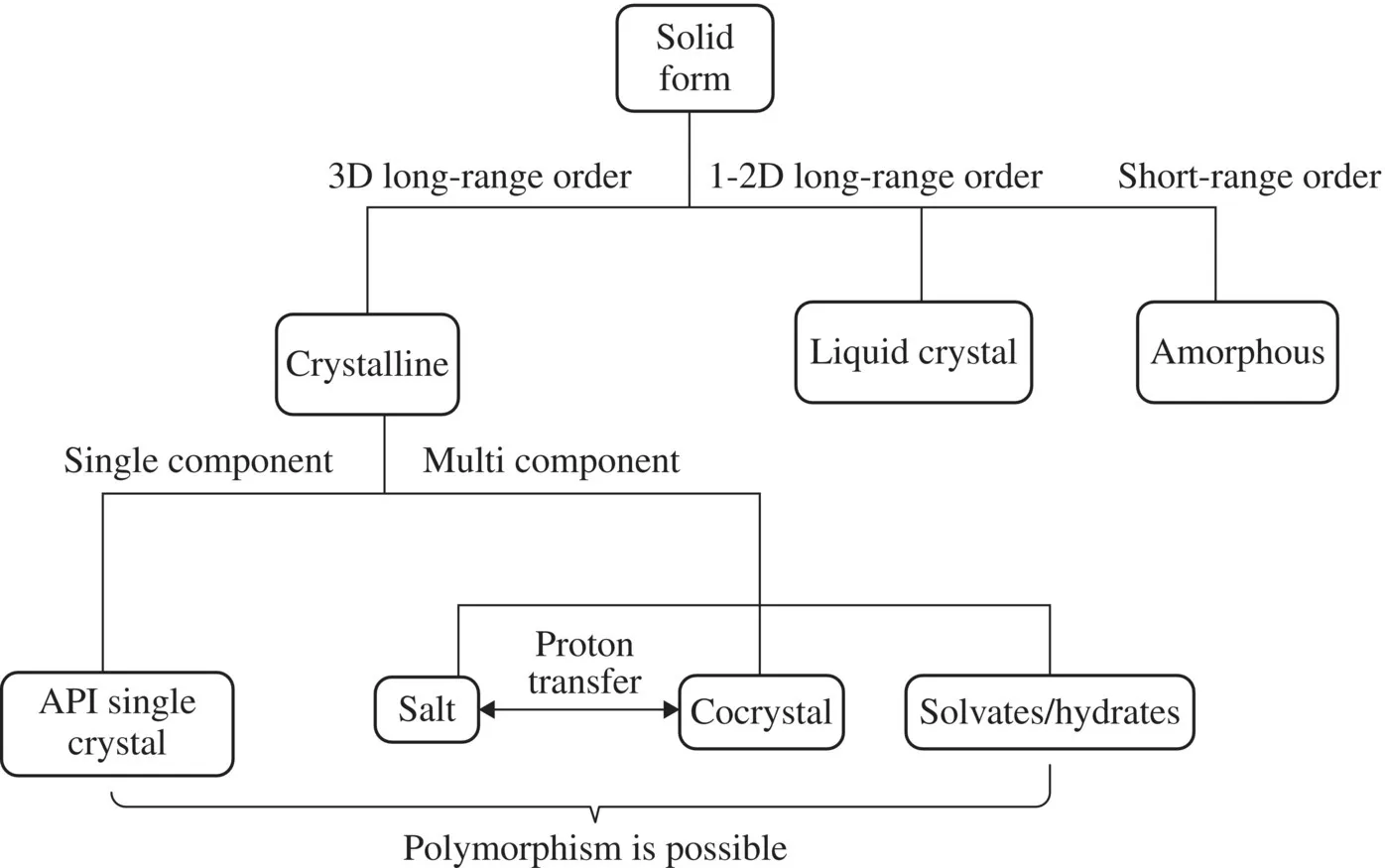
Figure 1.2 A typical pharmaceutical solid-state landscape.
Different crystalline structures of one substance (single- or multicomponent) are named polymorphs [9, 10]. Polymorphism, which exists as a result of different crystal packing of rigid molecules, is called a packing polymorphism. Conformational polymorphism is a more common phenomenon for typically flexible drug-like molecules and results from crystallization of different conformers of the same molecule. At a given environmental ...
Table of contents
- COVER
- TITLE PAGE
- TABLE OF CONTENTS
- LIST OF CONTRIBUTORS
- PREFACE
- EDITOR’S BIOGRAPHY
- 1 COMPUTATIONAL PHARMACEUTICAL SOLID-STATE CHEMISTRY
- 2 NAVIGATING THE SOLID FORM LANDSCAPE WITH STRUCTURAL INFORMATICS
- 3 THEORETICAL HYDROGEN-BONDING ANALYSIS FOR ASSESSMENT OF PHYSICAL STABILITY OF PHARMACEUTICAL SOLID FORMS
- 4 IMPROVING FORCE FIELD PARAMETERS FOR SMALL-MOLECULE CONFORMATION GENERATION
- 5 ADVANCES IN CRYSTAL STRUCTURE PREDICTION AND APPLICATIONS TO PHARMACEUTICAL MATERIALS
- 6 INTEGRATING COMPUTATIONAL MATERIALS SCIENCE TOOLS IN FORM AND FORMULATION DESIGN
- 7 CURRENT COMPUTATIONAL APPROACHES AT ASTRAZENECA FOR SOLID-STATE AND PROPERTY PREDICTIONS
- 8 SYNTHONIC ENGINEERING
- 9 NEW DEVELOPMENTS IN PREDICTION OF SOLID-STATE SOLUBILITY AND COCRYSTALLIZATION USING COSMO-RS THEORY
- 10 MODELING AND PREDICTION OF SOLID SOLUBILITY BY GE MODELS
- 11 MOLECULAR SIMULATION METHODS TO COMPUTE INTRINSIC AQUEOUS SOLUBILITY OF CRYSTALLINE DRUG-LIKE MOLECULES
- 12 CALCULATION OF NMR TENSORS
- 13 MOLECULAR DYNAMICS SIMULATIONS OF AMORPHOUS SYSTEMS
- 14 NUMERICAL SIMULATIONS OF UNIT OPERATIONS IN PHARMACEUTICAL SOLID DOSE MANUFACTURING
- INDEX
- END USER LICENSE AGREEMENT
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Computational Pharmaceutical Solid State Chemistry by Yuriy A. Abramov in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Pharmacology. We have over one million books available in our catalogue for you to explore.