
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Introduction to Heterocyclic Chemistry
About this book
A unique approach to a core topic in organic chemistry presented by an experienced teacher to students and professionals
Heterocyclic rings are present in the majority of known natural products, contributing to enormous structural diversity. In addition, they often possess significant biological activity. Medicinal chemists have embraced this last property in designing most of the small molecule drugs in use today. This book offers readers a fundamental understanding of the basics of heterocyclic chemistry and their occurrence in natural products such as amino acids, DNA, vitamins, and antibiotics. Based on class lectures that the author has developed over more than 40 years of teaching, it focuses on the chemistry of such heterocyclic substances and how they differ from carbocyclic systems.
Introductory Heterocyclic Chemistry offers in-depth chapters covering naturally occurring heterocycles; properties of aromatic heterocycles; ?-deficient heterocycles; ?-excessive heterocycles; and ring transformations of heterocycles. It then offers an overview of 1,3-dipolar cycloadditions before finishing up with a back-to-basics section on nitriles and amidines.
Introductory Heterocyclic Chemistry is an excellent text for undergraduate and graduate students as well as chemists in industrial environments in chemistry, pharmacy, medicinal chemistry, and biology.
Heterocyclic rings are present in the majority of known natural products, contributing to enormous structural diversity. In addition, they often possess significant biological activity. Medicinal chemists have embraced this last property in designing most of the small molecule drugs in use today. This book offers readers a fundamental understanding of the basics of heterocyclic chemistry and their occurrence in natural products such as amino acids, DNA, vitamins, and antibiotics. Based on class lectures that the author has developed over more than 40 years of teaching, it focuses on the chemistry of such heterocyclic substances and how they differ from carbocyclic systems.
Introductory Heterocyclic Chemistry offers in-depth chapters covering naturally occurring heterocycles; properties of aromatic heterocycles; ?-deficient heterocycles; ?-excessive heterocycles; and ring transformations of heterocycles. It then offers an overview of 1,3-dipolar cycloadditions before finishing up with a back-to-basics section on nitriles and amidines.
- Presents a conversational approach to a fundamental topic in organic chemistry teaching
- Offers a unique look at this core organic chemistry topic via important naturally occurring and/or biologically active heterocycles
- Based on the author's many years of class lectures for teaching at the undergraduate and graduate level as well as pharmaceutical-industry courses
- Clear, concise, and accessible for advanced students of chemistry to gain a fundamental understanding of the basics of heterocyclic chemistry
Introductory Heterocyclic Chemistry is an excellent text for undergraduate and graduate students as well as chemists in industrial environments in chemistry, pharmacy, medicinal chemistry, and biology.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Introduction to Heterocyclic Chemistry by Peter A. Jacobi in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Organic Chemistry. We have over one million books available in our catalogue for you to explore.
Information
1
Some Biologically Important Heterocycles of Nature
Heterocyclic rings come in many sizes and shapes, and they may be either aromatic or non‐aromatic, fused or non‐fused. This chapter provides a brief survey of some of the most biologically important heterocycles found in nature.
Let us begin our discussion with the three common amino acids tryptophan, proline, and histidine (Figure 1.1). Tryptophan contains an indole skeleton (blue), which is aromatic by virtue of having 10 π‐electrons in a cyclic conjugated array, two of which are donated by the ring nitrogen. Proline, on the other hand, is clearly non‐aromatic, since the pyrrolidine ring has only the free electron pair on nitrogen. But what about histidine, the distinguishing feature of which is an imidazole core with a total of eight electrons? Does this ring system satisfy Hückel's rule? The answer is yes, since one of the electron pairs resides in an orthogonal sp2‐orbital, leaving 6 π‐electrons to constitute the aromatic sextet. Lastly, while not an amino acid itself, we include in this introduction serotonin, a product of catabolism of tryptophan [1a]. The chief function of this indole alkaloid is as a neurotransmitter in the brain, and as such, it plays a key role in regulating mood. Drugs that alter the concentration of serotonin in the brain have found use in treating depression and anxiety disorders.
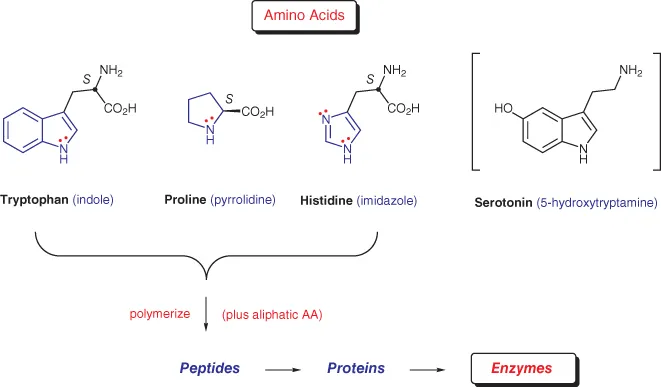
Figure 1.1 Some naturally occurring heterocycles.
As to their own biological role, amino acids are ubiquitous as the molecular building blocks of peptides, proteins, and enzymes. In addition to the three heterocyclic amino acids just described, 17 others make up the class of 20 naturally occurring amino acids, 9 of which are considered “essential” (i.e., they cannot be biosynthesized by humans, and must be provided by diet). In this context, one might wonder how nature functions at such a complex level with such a limited “tool chest.” Part of the answer is given by the simple equation N=20n, where N equals the number of possible peptides/proteins, 20 equals the number of monomer building blocks, and n equals the number of amino acids in the chain. The results can be staggering! For example, the number of structurally unique dipeptides would total 400; for n=5 this number jumps to 3,200,000; and for n=100, which is still quite a small protein, the possible combinations would be, well, astronomical (many orders of magnitude greater than the estimated number of atoms in the universe) [1b].
Proteins are one example of a class of compounds known as informational macromolecules, which control crucial life processes. We saw above how great complexity can be generated from a relatively small group of amino acid building blocks, even considering just the primary structure of the derived proteins. However, proteins are not unique in this capability, and it has been estimated that over 90% of all of the organic material found in living organisms, including many thousands of macromolecules, can be generated from about three dozen monomeric species [1a]. Of these, 20 constitute the naturally occurring amino acids. Another five are the DNA and RNA bases adenine (abbreviated A), thymine (T), guanine (G), and cytosine (C), all found in DNA, and uracil (U), which replaces thymine in RNA (Figure 1.2). A and G are examples of purine heterocycles, while T, C, and U are pyrimidines.
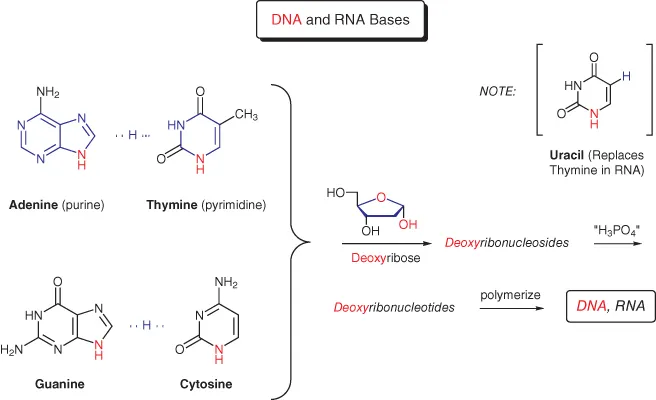
Figure 1.2 Naturally occurring heterocycles.
DNA is formally derived from A, T, G, and C by initial condensation of the NH groups shown in red with 2‐deoxyribose (an example of a furanose heterocycle, and another of nature's basic building blocks). The resultant deoxyribonucleosides then undergo phosphorylation at the primary hydroxyl group to afford the corresponding deoxyribonucleotides, which on polymerization lead to single stranded DNA. But this is not the end of the story. Most readers will be aware of the double helical nature of DNA, wherein base pairing through hydrogen bonding assures that identical concentrations of A and T will always be present. The same holds true for G and C. This observation was one of the keys to unraveling the structure of DNA, and it provided a molecular basis for the process of replication. RNA, produced from DNA by transcription, is a single stranded polynucleotide with uracil substituting for thymine, and ribose replacing deoxyribose. In the third step for processing genetic information, the message encoded in RNA is translated by ribosomes to that required for synthesizing specific proteins.
1.1 Vitamins
Who among us has not at some point been concerned with “getting enough vitamins?” Although generally required in only trace quantities, these micronutrients are one of five essential components of a healthy diet (the others being carbohydrates, fats, proteins, and certain mineral elements). Vitamins perform a myriad of biological functions, and they are generally classified as being either fat soluble or water soluble. All of the water‐soluble vitamins are heterocycles, a sampling of which are described below (Figure 1.3) [1].
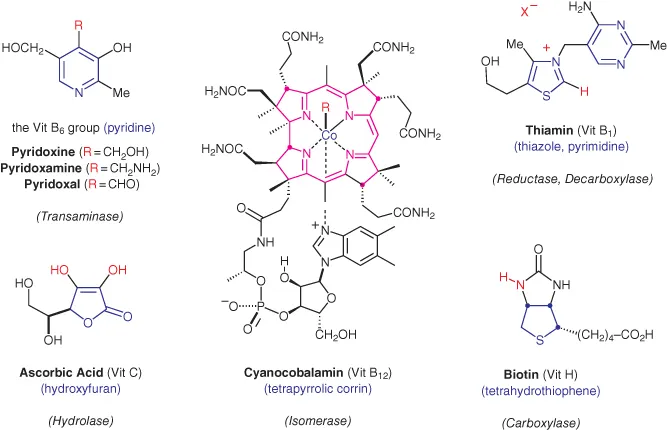
Figure 1.3 Heterocyclic vitamins.
Pyridoxine is one of a group of three closely related pyridine heterocycles that constitute the vitamin B6 group of vitamins (Figure 1.3). These species are best known for their role in transamination reactions, wherein an amino group from one α‐amino acid is reversibly transferred to the α‐carbon of an α‐keto acid. This process is coupled with the interconversion of pyridoxal and pyridoxamine, according to the overall equation:
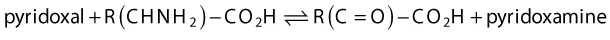
While the details for this enzyme catalyzed transformation are complex, we shall see later that each step finds precedent in common bond forming reactions employed in heterocycle synthesis.
Ascorbic acid (vitamin C) is best known as a preventive and curative agent for the debilitating disease scurvy, which is characterized by lethargy, brown spots on the skin and gums, open sores, and if left untreated, death from bleeding. Human beings are among the few vertebrates who cannot biosynthesize this substance from glucose. Up until the early 1800s scurvy was common among sailors and other adventurers lacking access to fresh citrus fruit, an excellent source of vitamin C. The British Navy is credited with making the observation that a daily ration of citrus juic...
Table of contents
- Cover
- Table of Contents
- Dedication
- Preface
- Acknowledgments
- Chapter 1: Some Biologically Important Heterocycles of Nature
- Chapter 2: Orbitals and Aromaticity; Chemical Reactivity
- Chapter 3: A Prelude to Synthesis
- Chapter 4: π‐Deficient Heterocycles: Some Physical Properties
- Chapter 5: π‐Deficient Heterocycles: De Novo Syntheses
- Chapter 6: π‐Deficient Heterocycles: Introduction of New Substituents: Nucleophilic Substitution
- Chapter 7: π‐Deficient Heterocycles: Introduction of New Substituents: Heterocyclic N‐Oxides
- Chapter 8: π‐Deficient Heterocycles: Introduction of New Substituents: Quinolines and Isoquinolines
- Chapter 9: π‐Deficient Heterocycles: Manipulation of Existing Substituents
- Chapter 10: π‐Excessive Heterocycles: General Properties
- Chapter 11: π‐Excessive Heterocycles: De Novo Syntheses
- Chapter 12: π‐Excessive Heterocycles: Introduction of New Substituents
- Chapter 13: Ring Transformations of π‐Excessive Heterocycles: Diels‐Alder Reactions
- Chapter 14: Heterocycles as Synthons
- Chapter 15: 1,3‐Dipolar Cycloadditions—An Overview
- Chapter 16: Back to Basics
- Chapter 17: A Brief Synopsis
- Index
- End User License Agreement