
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Operator's Guide to Process Compressors
About this book
The perfect primer for anyone responsible for operating or maintaining process gas compressors.
Gas compressors tend to be the largest, most costly, and most critical machines employed in chemical and gas transfer processes. Since they tend to have the greatest effect on the reliability of processes they power, compressors typically receive the most scrutiny of all the machinery among the general population of processing equipment. To prevent unwanted compressor failures from occurring, operators must be taught how their equipment should operate and how each installation is different from one another.
The ultimate purpose of this book is to teach those who work in process settings more about gas compressors, so they can start up and operate them correctly and monitor their condition with more confidence. Some may regard compressor technology as too broad and complex a topic for operating personnel to fully understand, but the author has distilled this vast body of knowledge into some key, easy to understand lessons for the reader to study at his or her own pace.
This groundbreaking new work is a must-have for any engineer, operator, or manager working with process compressors.
The main goals of this book are to:
- Explain important theories and concepts about gases and compression processes with a minimum of mathematics
- Identify key compressor components and explain how they affect reliability
- Explain how centrifugal compressors, reciprocating compressors, and screw compressors function.
- Explain key operating factors that affect reliabilityIntroduce the reader to basic troubleshooting methodologies
- Introduce operators to proven field inspection techniques
- Improve the confidence of personnel operating compressors by teaching them the basics of compressor theory
- Improve compressor reliability plantwide by teaching operating and inspection best practices
- Improve communication between operating and supporting plant personnel by providing a common vocabulary of compressor terms
- Help processing plants avoid costly failures by teaching operators how to identify early compressor issues during field inspections
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Chapter 1
Introduction to Gases
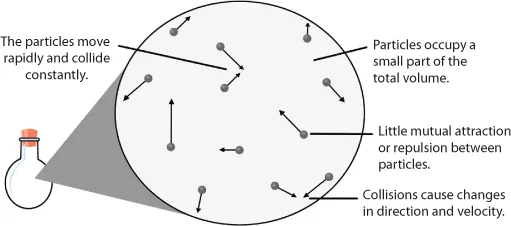
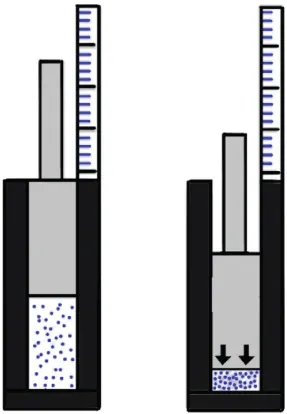
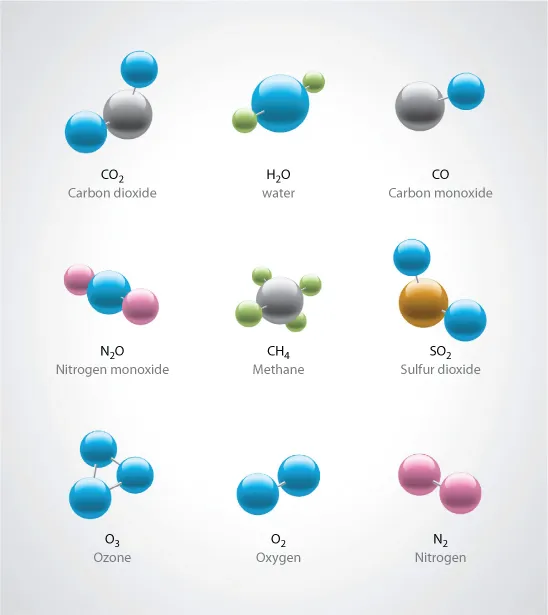
Table of contents
- Cover
- Title page
- Copyright page
- Dedication
- Acknowledgements
- Preface
- Chapter 1: Introduction to Gases
- Chapter 2: Commonly Used Compressor Flow Terms
- Chapter 3: Compression Processes
- Chapter 4: What Role the Compression Ratio Plays in Compressor Design and Selection
- Chapter 5: An Introduction to Compressor Operations
- Chapter 6: Centrifugal Compressors
- Chapter 7: How Process Changes Affect Centrifugal Compressor Performance
- Chapter 8: How to Read a Centrifugal Compressor Performance Map
- Chapter 9: Keeping Your Centrifugal Compressor Out of Harm’s Way
- Chapter 10: Troubleshooting Centrifugal Compressors in Process Services
- Chapter 11: Reciprocating Compressors
- Chapter 12: Troubleshooting Reciprocating Compressors in Process Services
- Chapter 13: Screw Compressors
- Chapter 14: Compressor Start-Up Procedures
- Chapter 15: Compressor Trains: Drivers, Speed Modifiers, and Driven Machines
- Chapter 16: Compressor Components
- Chapter 17: The Importance of Lubrication
- Chapter 18: Inspection Ideas for Operators and Field Personnel
- Chapter 19: Addressing Reciprocating Compressor Piping Vibration Problems: Design Ideas, Field Audit Tips, and Proven Solutions
- Chapter 20: Collecting and Assessing Piping Vibration
- Appendix A: Practice Problems Related to Chapters 1 Through 4 Topics
- Appendix B: Glossary of Compressor Technology Terms
- Index
- End User License Agreement
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app