eBook - ePub
Kinetics of Chemical Reactions
Decoding Complexity
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
This second, extended and updated edition presents the current state of kinetics of chemical reactions, combining basic knowledge with results recently obtained at the frontier of science.
Special attention is paid to the problem of the chemical reaction complexity with theoretical and methodological concepts illustrated throughout by numerous examples taken from heterogeneous catalysis combustion and enzyme processes.
Of great interest to graduate students in both chemistry and chemical engineering.
Special attention is paid to the problem of the chemical reaction complexity with theoretical and methodological concepts illustrated throughout by numerous examples taken from heterogeneous catalysis combustion and enzyme processes.
Of great interest to graduate students in both chemistry and chemical engineering.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Kinetics of Chemical Reactions by Guy B. Marin,Gregory S. Yablonsky,Denis Constales in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Physical & Theoretical Chemistry. We have over one million books available in our catalogue for you to explore.
Information
1
Introduction
1.1 Overview
Chemistry is a complex science, especially for chemists. The etymology of the word “chemistry,” the science of matter and its transformations, is a debatable issue [1]. It is very likely that it has been borrowed from the ancient name for Egypt, “Keme” the birthplace of alchemy. The word “complex” comes from the Latin word “complexus” the past participle of “complecti” (to entwine, encircle, compass, infold), from “com” (together) and “plectere” (to weave, braid).
Decoding complexity is considered one of the main scientific problems of the twenty‐first century. In chemistry, this process of decoding aims at explaining the temporal evolution of a multicomponent chemical mixture. In this book, depending on the context, there are three different meanings of “time”:
- “Clock” time , or astronomic time , or “external” time of the system, t : This time relates to the change of chemical composition observed during some time interval.
- “Internal” or “intrinsic” time: Typically, we consider this time when we are talking about the hierarchy of times of different chemical processes or reactions. For a first‐order reaction, the intrinsic time, that is, the timescale at which the reaction occurs, is the reciprocal value of its rate coefficient that has the dimension per second.
- Residence time: This time reflects the “transport time” of a chemical process, for example, in a plug‐flow reactor (PFR) (see Chapter 3).
An excellent collection of the different meanings of time can be found in Ref. [2].
Formally, the non‐steady‐state model for a chemical process in a closed system (batch reactor) is identical to the steady‐state model for the same chemical process in an open system in which the longitudinal profile of the chemical composition is taken into account, but the radial profile is neglected (see Chapter 3 for more details). In the latter model, the space time, which is proportional to the residence time, corresponds to astronomical time in the model for the batch reactor.
In the description of chemical complexity, the first key words are “many components,” “many reactions” and “change,” that is, a multicomponent chemical mixture changes in time and space. For example, in the homogeneous gas‐phase oxidation of hydrogen
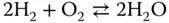
there are as much as nine different components and as much as 60 reactions involved. See Chapter 2 for more details.
In heterogeneous reactions, for example gas–solid reactions, the situation becomes even more complicated. Rephrasing Lewis Carroll's saying from Alice in Wonderland, “curiouser and curiouser,” one can say “complexier and complexier.” Over 90% of industrial chemical reactions occur with solid catalysts that can dramatically accelerate these reactions. Many catalysts are multicomponent solids, for example, mixed transition metal oxides on some support used in the selective oxidation of hydrocarbons. Catalysts can exist in different states that depend on the oxidation degree, water content, bulk structure, and so on. These states have different physicochemical properties and different abilities to accelerate reactions. Moreover, the catalyst composition changes in time under the influence of the reaction medium. This is the level of chemical complexity that needs to be decoded.
1.2 Decoding Complexity in Chemical Kinetics
Immediately, many questions regarding this decoding arise:
- What are we going to decode?
- Based on which experimental characteristics are we going to decode?
- In which terms are we going to decode?
In this book, our answers are the following:
- We are going to decode data mostly related to heterogeneous catalytic reactions.
- We are going to decode these data based on experimental characteristics obtained during kinetic experiments, that is, measurements of rates of transformation of chemical components.
- We are going to try and interpret these kinetic data based on the concept of reaction mechanism (or detailed mechanism), a detailed description of the steps leading from reactants to products of the reaction, which includes intermediates.
We consider this decoding to be an inherent feature of chemical kinetics, which can be defined as the science of rates and mechanisms of chemical reactions. One can hardly overestimate the role of chemical kinetics, both in understanding the “generative” character of chemical reactions and in designing new chemical processes and reactors.
1.3 Three Types of Chemical Kinetics
Presently, chemical kinetics is an area comprising challenges and adventures, in which at least four sciences overlap: chemistry, physics, chemical engineering, and mathematics. In fact, contemporary chemical kinetics itself is a complex combination of different areas. Depending on the goal of a kinetic analysis, one may distinguish between applied kinetics, detailed kinetics, and mathematical kinetics.
1.3.1 Applied Kinetics
The goal of applied kinetics is to obtain kinetic dependences for the design of efficient catalytic processes and reactors. Kinetic dependences are dependences of the rates of chemical transformations on reaction conditions, that is, t...
Table of contents
- Cover
- Table of Contents
- Preface to First Edition
- Preface to Second Edition
- 1 Introduction
- 2 Chemical Reactions and Complexity
- 3 Kinetic Experiments: Concepts and Realizations
- 4 Chemical Book‐keeping: Linear Algebra in Chemical Kinetics*
- 5 Steady‐State Chemical Kinetics: A Primer
- 6 Steady‐state Chemical Kinetics: Machinery
- 7 Linear and Nonlinear Relaxation: Stability
- 8 Nonlinear Mechanisms: Steady State and Dynamics
- 9 Kinetic Polynomials
- 10 Temporal Analysis of Products: Principles, Applications, and Theory
- 11 Joint Kinetics
- 12 Decoding the Past
- 13 Decoding the Future
- Index
- End User License Agreement