
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
Using classification, diagrams and crystallography elements, we describe in this book the bonds in the crystals using the basic patterns. The use of various criteria such as ionicity character of the bonds, the use of hard sphere models, the Pauling rules and the spatial availability of ions all together make it possible to better understand the spatial organization of typical crystals. Through original representations, the structure and the nature of the bonds in binary crystals of MX- and MX2- types as well as the ternary crystals of the perovskite and spinel type are studied.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Inorganic Chemistry by Robert Valls in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Inorganic Chemistry. We have over one million books available in our catalogue for you to explore.
Information
1
Knowledge of the Periodic Table
1.1. Presentation of the periodic table
The periodic table is one of the chemist’s everyday tools for retrieving the properties of an element based on its position in this table and the various values associated with it. However, before proceeding to use it, knowledge of several simple keys and of their limits should be acquired and their operation should be mastered.
This table should not be perceived as a visionary anticipation or thought of as being received by a scientist through revelation. It is the result of lengthy and patient efforts sustained by Mendeleev and all the chemists in his time, drawing on the empirical knowledge accumulated throughout the 18th and 19th Centuries by several generations of chemists engaged in the pursuit of this classification, which was supposed to name and organize all the elements. Dozens of scientists should be cited, but in order to keep the presentation simple, only three of them will be mentioned here, namely those whose interventions were essential. The first one was Lavoisier, who in 1789 had formalized the notion of elements, then came Mendeleev, who is recognized as the creator of the periodic table according to his work published in 1869, and finally Moseley, who in 1913 offered the key to the complete form of the periodic table.
The use of the table in the absence of several reading keys may prove discouraging, as it requires great memory and leaves the impression of a series of successive specific cases. Moreover, the method used by chemists in order to memorize the periodic table should differ from the one employed for multiplication tables (it is less mechancal), similar to that applied for memorizing the names, location and characteristics of places encountered every day and which are part of real-life experience, therefore involving every day and which are part of real-life experience, therefore involving
Resorting to deductive presentations of the periodic table does not simplify its acquisition, since the table, though following nearly mathematical laws for some characteristics of the elements, represents a synthesis of all the diversity of nature when it comes to other characteristics.
1.2. Construction of the periodic table
1.2.1. History
In order to allow for a better grasp of the subject, a brief history of the periodic table will be presented, covering five periods that are in our opinion decisive for its elaboration, and whose landmarks are given by the following three important names: Lavoisier, Mendeleev and Moseley.
In the period from Antiquity to the Renaissance, seven metals were known: gold, silver, mercury, copper, iron, lead and tin, as well as several other elements such as sulfur, antimony, arsenic, carbon and phosphorus. By the end of the 17th Century, only these 12 simple substances had been discovered.
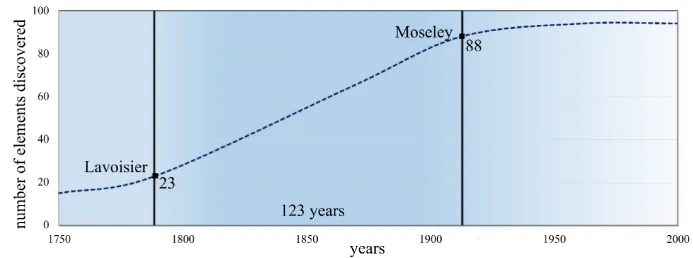
Figure 1.1. Discovery of elements throughout the centuries
As the techniques used for analysis evolved, Lavoisier had 23 elements at the beginning of his research work, but their number rapidly increased, so that in 1869 Mendeleev gathered 65 elements in an arrangement that he proposed as the first classification, and then in 1913 Moseley gave the final form of the current periodic table, which contained 88 elements (see Figure 1.1).
1.2.1.1. The pioneer: Antoine-Laurent de Lavoisier
In 1789 Lavoisier (1743–1794), with his Elements of chemistry, revolutionized (an action fit for those times) chemistry, being the first to propose a distinction between simple and compound substances. He also advanced the idea that certain chemical products, which cannot be decomposed, should be considered elements. It is safe to consider him the founding father of modern chemistry, given his systematic use of weighing instruments. Moreover, he set the bases of chemistry in the following terms:
“To solve these two questions, it is necessary to be previously acquainted with the analysis of the fermentable substance, and of the products of the fermentation. We may lay it down as an incontestable axiom, that, in all the operations of art and nature, nothing is created; an equal quantity of matter exists both before and after the experiment; the quality and quantity of the elements remain precisely the same; and nothing takes place beyond changes and modifications in the combination of these elements. Upon this principle the whole art of performing chemical experiments depends: We must always suppose an exact equality between the elements of the body examined and those of the products of its analysis.” (Antoine-Laurent de Lavoisier, Elements of Chemistry, 1789)
A very reductionist but quite effective variant of the above fragment is often stated as follows: Nothing is created, nothing is lost, everything is transformed. Lavoisier proposed the idea of simple substances (elements), while he was still far from the periodic table, but he gradually created a ranking (see Table 1.1).
Table 1.1. List of simple substances propos...
Table of contents
- Cover
- Table of Contents
- Title
- Copyright
- Acknowledgments
- Introduction
- 1 Knowledge of the Periodic Table
- 2 Knowledge of Metallic Crystals
- 3 Knowledge of Ionic Crystals
- Appendix: Ionic Radii
- Bibliography
- Index
- End User License Agreement