
- English
- ePUB (mobile friendly)
- Available on iOS & Android
About this book
This new volume provides a timely study on the environmental challenges from a specific class of perfluorinated chemical compounds (PFCs) that are now being recognized as a worldwide health threat. Recent studies report that levels of classes of PFCs known as polyfluoroalkyl and perfluoroalkyl (PFASs) exceed federally recommended safety levels in public drinking-water supplies for 6 million people in the United States and that as many as 100 million people could be at risk from exposure to these chemicals.
These chemicals occur globally in wildlife and humans. Both PFCAs and PFSAs have been produced for more than 50 years, but have only become of interest to regulators and environmentalists since the late 1990s. Recent advances in analytical methodology has enabled widespread detection in the environment and humans at trace levels. These toxic chemicals have been found in outdoor and indoor air, surface and drinking water, house dust, animal tissue, human blood serum, and human breast milk. Of great concern to communities is the presence of these compounds in a number of drinking water supplies in the U.S. and other countries.
This new volume provides a timely explanation of the chemicals, provides a detailed review of the regulations both in the US and European Community, explains the health risk literature, and then explores in great detail available treatment technologies. The volume is a must for public water supply facilities, industrial operations that have historically used these chemicals and face legacy pollution issues, policy makers and the general public.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
Chapter 1
What Fluoropolymers Are
1.1 Introduction
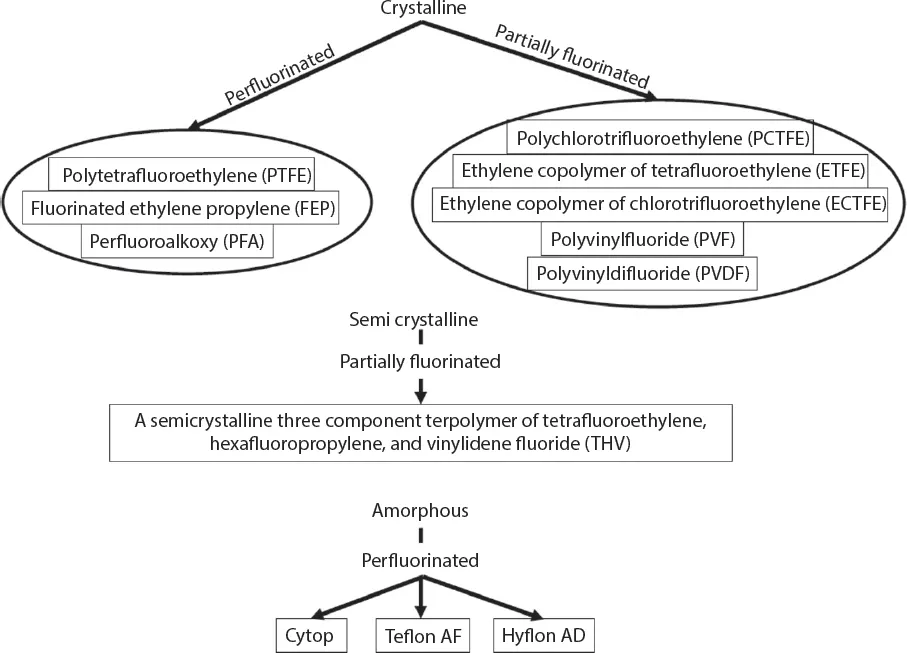
1.2 Evolution of Fluoropolymers and the Markets
| 1886 | Henri Moisson isolated elemental fluorine, for which he received the Nobel Prize in Chemistry. |
| 1890s | SbF3 is applied in a Cl/F exchange reaction to prepare fluorinated aromatics and the first chlorofluorocarbon gas (CF2Cl2). |
| 1931 | General Motors, in partnership with E. I du Pont de Nemours & Co., formed a new corporation, Kinetic Chemicals Inc., to produce commercial quantities of the trademarked product Freon-12. |
| 1930s | Several other Freons were developed, including Freon-114 (CClF2CClF2) a precursor of tetrafluoroethylene (TFE). |
| 1934 | The first patent for a fluoropolymer was filed by IG-Farbenindustrie in Hoechst/Frankfurt, in Germany. |
| 1938 | Roy Plunkett, a DuPont chemist working on new types of Freons, independently discovered PTFE (Teflon) while attempting to chlorinate gaseous TFE. |
| 1949 | DuPont introduces Teflon. Plunkett began working for DuPont Jackson Laboratory in Deepwater, N.J., as a research chemist in 1936. Plunkett’s discovery was found to be both heat-resistant and stick-resistant. After 10 years of research, DuPont introduced Teflon in 1949. |
| Late 1940s | 3M purchases the Simon Electrofluorination Patent. Electrochemical fluorination (ECF), or electrofluorination, is a foundational organofluorine chemistry method for the preparation of fluorocarbon-based organofluorine compounds. The general approach represents an application of electrosynthesis. The fluorinated chemical compounds produced by ECF are useful because of their distinctive solvation properties and the relative inertness of carbon–fluorine bonds. Two ECF synthesis routes are commercialized and commonly applied, the Simons Process and the Phillips Petroleum Process. Additionally, it is also possible to electrofluorinate in various organic media. Prior to the development of the Simon method, fluorination with fluorine, a dangerous oxidant, was a dangerous and wasteful process. Also, ECF can be cost effective, but it may also result in low yields. |
| 1953 | Kellog Co. introduced polychlorotrifluoroethylene (PCTFE) under the trade name Kel-F 81. PCTFE, a homopolymer of CTFE, contained chlorine in the fluoropolymer backbone making it a more processable alternative to PTFE. |
| 1956 | 3M begins selling Scotchgard Protector. Scotchgard Protector contained a fluorochemical that helped it repel stains. |
| 1960 | FEP (fluorinated ethylene propylene), the first copolymer of TFE was introduced. |
| 1961 | Dupont released polyvinylfluoride (PVF) which contained only one fluorine in the ethylene monomer unit, ... |
Table of contents
- Cover
- Title page
- Copyright page
- Preface
- About the Author
- Abbreviations and Acronyms
- Useful Conversion Factors
- Chapter 1: What Fluoropolymers Are
- Chapter 2: Definitions, Uses, and Evolution of PFCs
- Chapter 3: Fire Fighting Foams
- Chapter 4: Health Risk Studies
- Chapter 5: Overview of the Environmental Concerns
- Chapter 6: The Supply Chain and Pathways to Contamination
- Chapter 7: Standards, Advisories, and Restrictions
- Chapter 8: Overview of Water Treatment Technology Options
- Chapter 9: Adsorption Technology
- Chapter 10: Case Studies
- Index
- End User License Agreement