
Novel Carbon Materials and Composites
Synthesis, Properties and Applications
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Novel Carbon Materials and Composites
Synthesis, Properties and Applications
About this book
Connects knowledge about synthesis, properties, and applications of novel carbon materials and carbon-based composites
This book provides readers with new knowledge on the synthesis, properties, and applications of novel carbon materials and carbon-based composites, including thin films of silicon carbide, carbon nitrite, and their related composites. It examines the direct bottom-up synthesis of the carbon-based composite systems and their potential applications, and discusses the growth mechanism of the composite structures. It features applications that range from mechanical, electronic, chemical, biochemical, medical, and environmental to functional devices.
Novel Carbon Materials and Composites: Synthesis, Properties and Applications covers an overview of the synthesis, properties, and applications of novel carbon materials and composites. Especially, it covers everything from chemical vapor deposition of silicon carbide films and their electrochemical applications to applications of various novel carbon materials for the construction of supercapacitors to chemical vapor deposition of diamond/silicon carbide composite films to the covering and fabrication processes of nanodot composites.
- Looks at the recent progress and achievements in the fields of novel carbon materials and composites, including thin films of silicon carbide, carbon nitrite, and their related composites
- Discusses the many applications of carbon materials and composites
- Focuses on the hot topic of the fabrication of carbon-based composite materials and their abilities to extend the potential applications of carbon materials
- Published as a title in the new Wiley book series Nanocarbon Chemistry and Interfaces.
Novel Carbon Materials and Composites: Synthesis, Properties and Applications is an important book for academic researchers and industrial scientists working in the fabrication and application of carbon materials and carbon-based composite materials and related fields.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
1
Cubic Silicon Carbide: Growth, Properties, and Electrochemical Applications
1.1 General Overview of Silicon Carbide
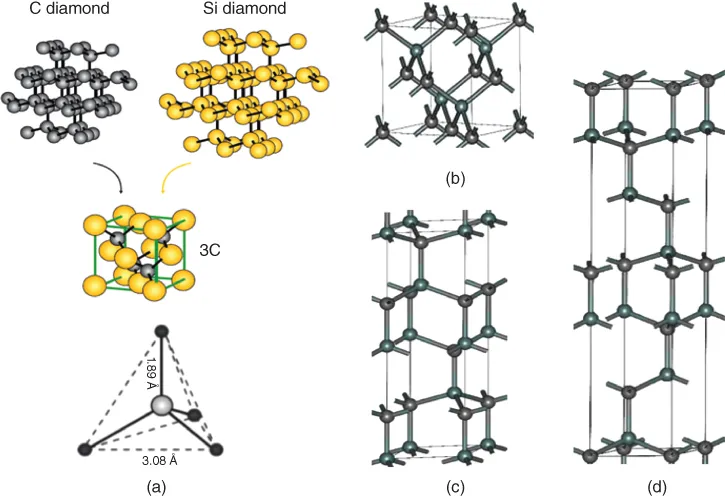
1.1.1 SiC Properties
| Property | 4H‐SiC | 6H‐SiC | 3C‐SiC | Si | Diamond |
| Energy bandgap at 300 K (eV) | 3.20 | 3.00 | 2.29 | 1.12 | 5.45 |
| Intrinsic carrier concentration at 300 K (cm−3) | 5 × 10−9 | 1.6 × 10−6 | 1.5 × 10−1 | 1 × 1010 | ∼10−27 |
| Critical breakdown electric field (MV cm−1) | 2.2 | 2.5 | 2.12 | 0.25 | 1–10 |
| Saturated electron drift velocity (×107 cm s−1) | 2.0 | 2.0 | 2.5 | 1.0 | 1.5 |
| Electron mobility (cm2 V−1 s−1) | 1000 | 600 | 800 | 1450 | 480 |
| Electron mobility (cm2 V−1 s−1) | 115 | 100 | 40 | 470 | 1600 |
| Thermal conductivity at 300 K (W cm−1 K−1) | 3.7 | 3.6 | 3.6 | 1.49 | 6–20 |
| Coefficient of thermal expansion at 300 K (10−6 K−1) | 4.3 4.7 | 4.3 4.7 | 3.2 | 3.0 | 1.0 |
| Lattice coefficient (a, c in Å) | a = 3.073 c = 10.053 | a = 3.081 c = 15.117 | a = 4.360 | a = 5.430 | a = 3.567 |
| Calculated elastic coefficient (GPa) | C44 = 600 | C11 = 500 C12 = 92 C44 = 168 | C11 = 352 C12 = 12 C44 = 233 | C11 = 167 C12 = 65 C44 = 80 | C11 = 1079 C12 = 124 C44 = 578 |
Table of contents
- Cover
- Table of Contents
- List of Contributors
- Series Preface
- Preface
- 1 Cubic Silicon Carbide: Growth, Properties, and Electrochemical Applications
- 2 Application of Silicon Carbide in Photocatalysis
- 3 Application of Silicon Carbide in Electrocatalysis
- 4 Carbon Nitride Fabrication and Its Water‐Splitting Applications
- 5 Carbon Materials for Supercapacitors
- 6 Diamond/β‐SiC Composite Films
- 7 Diamond/Graphite Nanostructured Film: Synthesis, Properties, and Applications
- 8 Carbon Nanodot Composites: Fabrication, Properties, and Environmental and Energy Applications
- Index
- End User License Agreement