- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
Combining theoretical knowledge of synthetic transformations, practical considerations, structural elucidation by interpretation of spectroscopic data as well as rationalization of structure-property relations, this textbook presents a series of 16 independent exercises, including detailed descriptions of experimental procedures, questions, and answers. The experimental descriptions are very helpful for guiding less experienced students towards a better understanding of practical aspects in synthetic organic chemistry, while the broad scope of the questions and answers is excellent for learning purposes. The exercises are based on published research articles, adapted for didactic purposes, and will thus inspire students by way of having to solve real-life problems in chemistry.
A must-have for MSc and PhD students as well as postdocs in organic chemistry and related disciplines, and lecturers and organizers of lab courses in organic chemistry.
A must-have for MSc and PhD students as well as postdocs in organic chemistry and related disciplines, and lecturers and organizers of lab courses in organic chemistry.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Chapter 1
Atovaquone: An Antipneumocystic Agent
Atovaquone is a pharmaceutical compound marketed in the United States under different combinations to prevent and treat pneumocystosis and malaria. In a report from 2012, a team of researchers described a novel synthetic process scalable to 200 kg, starting from isochromandione 1 and aldehyde 2 (Scheme 1.1) [1, 2].
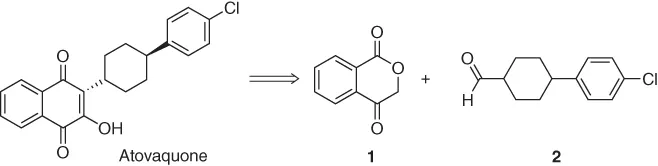
Scheme 1.1
The route to 1 is described in Scheme 1.2. A mixture of phthalic anhydride 3 and Et3N (1.07 equiv.) heated at 80 °C is treated over 4 h by portions of malonic acid (1.2 equiv.) and maintained at 80 °C for 10 h. Gas evolution was observed all along that period.1 After adding an excess of aq. HCl solution and cooling the mixture to 25 °C, the solid is filtered off and dried to afford acid 5 in 67% yield. This transformation presumably occurs through intermediate 4, having the molecular formula C10H8O5 and containing two carboxylic acid groups [3, 4].
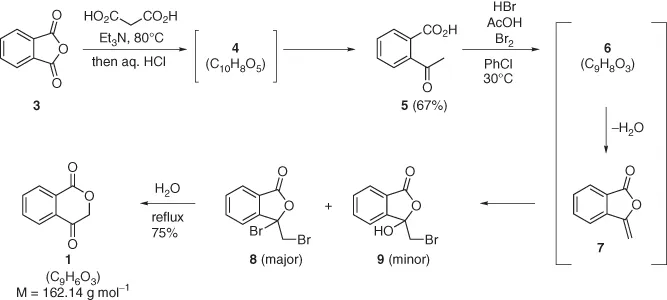
Scheme 1.2
Question 1.1
- Write the structure of 4 and suggest a plausible mechanism for its formation.
Question 1.2
- Suggest a plausible mechanism for the formation of 5 from 4.
A solution of 5 in chlorobenzene is reacted for 3 h at 30 °C in the presence of HBr (0.05 equiv.) and Br2 (1 equiv.) in acetic acid. This reaction leads to the formation of intermediate 6 (molecular formula C9H8O3) undergoing loss of a molecule of water to give intermediate 7, transformed into lactones 8 and 9 under reaction conditions. Water is then added, and the mixture is refluxed for 3 h and cooled to 60 °C. The organic layer is removed, the aqueous layer is extracted with chlorobenzene, and the combined organic layers are concentrated under reduced pressure. Addition of i-PrOH followed by cooling to 0 °C results in the formation of a solid, which is filtered, washed with i-PrOH, and dried to afford 1 in 75% yield.
Question 1.3
- Write the structure of 6 and suggest a plausible mechanism for its formation from 5 and its transformation into 7.
Question 1.4
- Suggest a plausible mechanism for the formation of 1 from 8 and 9.
Question 1.5
- The 1H-NMR spectra reported for compou...
Table of contents
- Cover
- Title Page
- Copyright
- Dedication
- Table of Contents
- Preface
- List of Abbreviations
- Chapter 1: Atovaquone: An Antipneumocystic Agent
- Chapter 2: SEN794: An SMO Receptor Antagonist
- Chapter 3: Synthesis of an H1–H3 Antagonist
- Chapter 4: Synthesis of Eletriptan
- Chapter 5: Total Synthesis and Structure Revision of Streptophenazine A
- Chapter 6: Synthesis of Leiodermatolide, A Biologically Active Macrolide
- Chapter 7: Azobenzene-Thiourea Catalysts for the Control of Chemical Reactivity with Light
- Chapter 8: Synthesis and Properties of a Photo-activatable Mimic of Pyridoxal 5ʹ-Phosphate
- Chapter 9: Fluorescent Peptides for Monitoring Activity of Autophagy-Initiating Enzyme
- Chapter 10: Fluorescent Peptide Probes for Cathepsin B
- Chapter 11: Total Synthesis of Stemoamide
- Chapter 12: Total Synthesis and Structure Revision of Caraphenol B
- Chapter 13: Synthetic Routes Toward Muricatacin and Analogs
- Chapter 14: Asymmetric Synthesis of (−)-Martinellic Acid
- Chapter 15: Cyclic Pseudopeptides as Potent Integrin Antagonists
- Chapter 16: Enantioselective Synthesis of Nonnatural Amino Acids for Incorporation in Antimicrobial Peptides
- Further Reading
- Index
- End User License Agreement
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Multi-Step Organic Synthesis by Nicolas Bogliotti,Roba Moumné in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Physical & Theoretical Chemistry. We have over one million books available in our catalogue for you to explore.