
eBook - ePub
Available until 6 Dec |Learn more
Solid-State Properties of Pharmaceutical Materials
This book is available to read until 6th December, 2025
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Available until 6 Dec |Learn more
Solid-State Properties of Pharmaceutical Materials
About this book
Presents a detailed discussion of important solid-state properties, methods, and applications of solid-state analysis
- Illustrates the various phases or forms that solids can assume and discussesvarious issues related to the relative stability of solid forms and tendencies to undergo transformation
- Covers key methods of solid state analysis including X-ray powder diffraction, thermal analysis, microscopy, spectroscopy, and solid state NMR
- Reviews critical physical attributes of pharmaceutical materials, mainly related to drug substances, including particle size/surface area, hygroscopicity, mechanical properties, solubility, and physical and chemical stability
- Showcases the application of solid state material science in rational selection of drug solid forms, analysis of various solid forms within drug substance and the drug product, and pharmaceutical product development
- Introduces appropriate manufacturing and control procedures using Quality by Design, and other strategies that lead to safe and effective products with a minimum of resources and time
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Solid-State Properties of Pharmaceutical Materials by Stephen R. Byrn,George Zografi,Xiaoming (Sean) Chen in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Industrial & Technical Chemistry. We have over one million books available in our catalogue for you to explore.
Information
1
SOLID-STATE PROPERTIES AND PHARMACEUTICAL DEVELOPMENT
1.1 INTRODUCTION
Solid-state chemistry and the solid-state properties of pharmaceutical materials play an ever increasing and important role in pharmaceutical development. There is much more emphasis on physical characterization since the release of the International Committee on Harmonization (ICH) Q6A guidance on specifications. This guidance directs the scientist to determine what solid form is present in the drug substance (active pharmaceutical ingredient [API]) and drug product. It directs the manufacturer to “know what they have.” Additionally, the ICH Q8 guidance on development and the ICH Q9 guidance on risk management require a firm understanding of how the medicine was developed and any risks involved.
There are many more poorly soluble drugs under development. In many cases, the solid form of the API and the solid form and formulation in the drug product determine apparent solubility that in turn determines blood levels. That is, the formulation determines bioavailability and therapeutic response. In these cases, it is even more important to physically characterize the API form and the formulations. Furthermore, the vast majority of medicines (drug products) are solids and those drug products that are not solids often start with solid APIs. In addition to solubility and bioavailability, the solid form may affect stability, flow, compression, hygroscopicity, and a number of other properties.
This book focuses on solid-state properties of pharmaceutical materials and methods of determining these properties. The authors have made every effort to include examples and case studies in order to illustrate the importance of knowing what you have. This book will focus on solid-state properties and general strategies for physical characterization. Case studies and practical examples will be emphasized. In many respects, this book will illustrate that a medicine is more than a molecule. Additional goals include providing a full physical/analytical/operational definition of the different solid forms as well as other terms frequently used in pharmaceutical materials science including: polymorph, solvate, amorphous form, habit, nucleation, transformation, dissolution, solubility, and stability.
1.2 SOLID-STATE FORMS
Pharmaceutical materials can exist in a crystalline or amorphous state. Figure 1.1 illustrates the crystalline state as a perfectly ordered solid with molecules (circles) packed in an orderly array. Figure 1.1 illustrates an amorphous material as a disordered material with only short-range order. Crystalline materials give an X-ray diffraction pattern because Bragg planes exist in the material (see Figure 1.2). Amorphous materials do not give a diffraction pattern (Figure 1.2). Of course, there are many interesting cases where a pharmaceutical material shows an intermediate degree of order falling somewhere between the highly ordered crystalline state and the disordered amorphous state. From a thermodynamic point of view, crystalline materials are more stable but the rate of transformation of amorphous materials to crystalline materials can be highly variable [1].
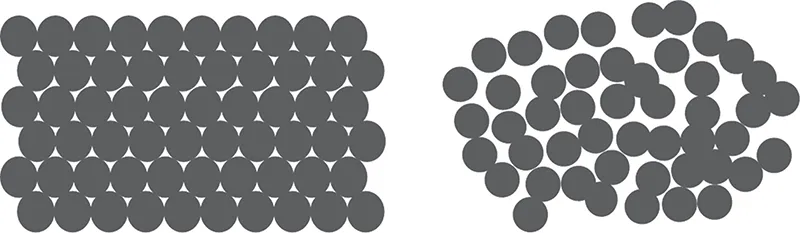
FIGURE 1.1 Idealized view of crystalline (left panel) and amorphous (right panel) material. In this two-dimensional figure, the molecules are viewed as circles.
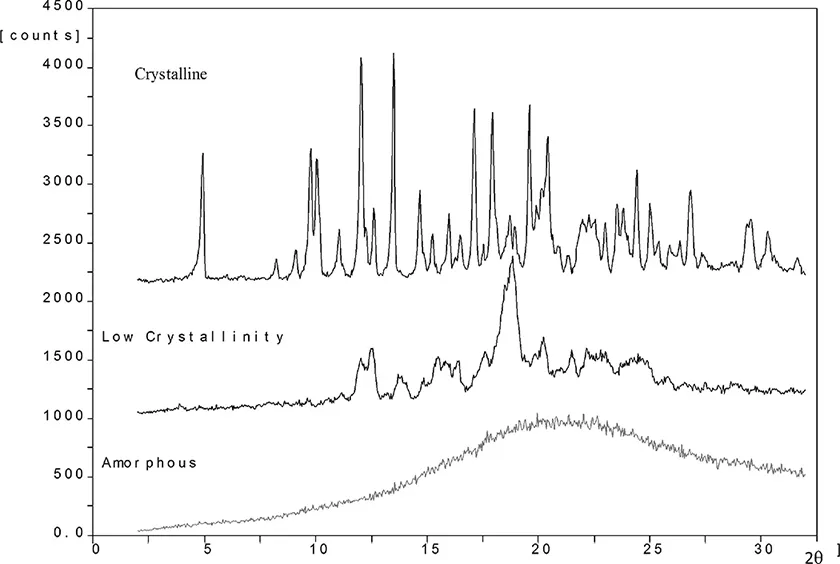
FIGURE 1.2 X-ray diffraction pattern of three samples, crystalline, low crystallinity, and amorphous.
Crystals of a pharmaceutical material from different sources can vary greatly in their size and shape. Typical particles in different samples may resemble, for example, needles, rods, plates, and prisms. Such differences in shape are collectively referred to as differences in morphology. This term merely acknowledges the fact of different shapes. It does not distinguish among the many possible reasons for the different shapes. Naturally, when different compounds are involved, different crystal shapes would be expected as a matter of course. When batches of the same substance display crystals with different morphology, however, further work is needed to determine whether the different shapes are indicative of polymorphs, solvates, or just habits. Because these distinctions can have a profound impact on drug performance, their careful definition is very important to our discourse. At this time, only brief definitions are presented.
- Polymorphs: When two crystals have the same chemical composition but different internal structure (molecular packing), they are polymorphic modifications, or polymorphs (think of the three forms of carbon: diamond, graphite, and fullerenes). Polymorphs can result from different molecular packing, different molecular conformation, different tautomeric structure, or combinations of these.
- Solvates: These crystal forms, in addition to containing molecules of the same given substance, also contain molecules of solvent regularly incorporated into a unique structure (think of wet, setting plaster: CaSO4 + 2H2O → CaSO4·2H2O).
- Habits: Crystals are said to have different habits when samples have the same chemical composition and the same crystal structure (i.e., the same polymorph and unit cell) but display different shapes (think of snowflakes).
Together, these solid-state physical modifications of a compound are referred to as crystalline forms. When differences between early batches of a substance are found by microscopic examination, for example, a reference to “form” is particularly useful in the absence of information that allows the more accurate description of a given variant batch (i.e., polymorph, solvate, habit, or amorphous material). The term pseudopolymorphism is applied frequently to designate solvates. These solid-state modifications have different physical properties.
To put these important definitions into a practical context, we consider two cases (aspirin and flufenamic acid) in which a drug was crystallized from several different solvents and different-shaped crystals resulted in each experiment. Although sometimes dramatically different shapes were obtained upon changing solvents for the various crystallizations, the final interpretations in the two cases are different. For aspirin, X-ray powder diffraction showed that all crystals regardless of shape had the same diffraction pattern. Thus, the different shaped crystals are termed crystal habits. For flufenamic acid, the different shaped crystals had different X-ray powder diffraction patterns. Subsequent analysis showed that the crystals did not contain solvent. Thus these different crystals are polymorphs.
Further analysis of the crystals from this case provides the single crystal structure. The single crystal structure gives the locations of the atoms r...
Table of contents
- Cover
- Title Page
- Copyright
- Preface
- Acknowledgments
- 1 Solid-State Properties and Pharmaceutical Development
- 2 Polymorphs
- 3 Solvates and Hydrates
- 4 Pharmaceutical Salts
- 5 Pharmaceutical Cocrystals
- 6 Amorphous Solids
- 7 Crystal Mesophases and Nanocrystals
- 8 X-Ray Crystallography and Crystal Packing Analysis
- 9 X-Ray Powder Diffraction
- 10 Differential Scanning Calorimetry and Thermogravimetric Analysis
- 11 Microscopy
- 12 Vibrational Spectroscopy
- 13 Solid-State NMR Spectroscopy
- 14 Particle and Powder Analysis
- 15 Hygroscopic Properties of Solids
- 16 Mechanical Properties of Pharmaceutical Materials
- 17 Solubility and Dissolution
- 18 Physical Stability of Solids
- 19 Chemical Stability of Solids
- 20 Solid-State Properties of Proteins
- 21 Form Selection of Active Pharmaceutical Ingredients
- 22 Mixture Analysis
- 23 Product Development
- 24 Quality by Design
- Index
- EULA