
Acid Gas Extraction for Disposal and Related Topics
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Acid Gas Extraction for Disposal and Related Topics
About this book
This is the fifth volume in a series of books focusing on natural gas engineering, focusing on the extraction and disposal of acid gas. This volume includes information for both upstream and downstream operations, including chapters on modeling, carbon capture, chemical and thermodynamic models, and much more.
Written by some of the most well-known and respected chemical and process engineers working with natural gas today, the chapters in this important volume represent the most cutting-edge and state-of-the-art processes and operations being used in the field. Not available anywhere else, this volume is a must-have for any chemical engineer, chemist, or process engineer working with natural gas.
There are updates of new technologies in other related areas of natural gas, in addition to the extraction and disposal of acid gas, including testing, reservoir simulations, acid gas injection, and natural gas hydrate formations. Advances in Natural Gas Engineering is an ongoing series of books meant to form the basis for the working library of any engineer working in natural gas today. Every volume is a must-have for any engineer or library.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
Chapter 1
Rate-Base Simulations of Absorption Processes; Fata Morgana or Panacea?
Abstract
1.1 Introduction
1.2 Procede Process Simulator (PPS)
1.3 Mass Transfer Fundamentals
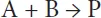
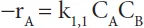
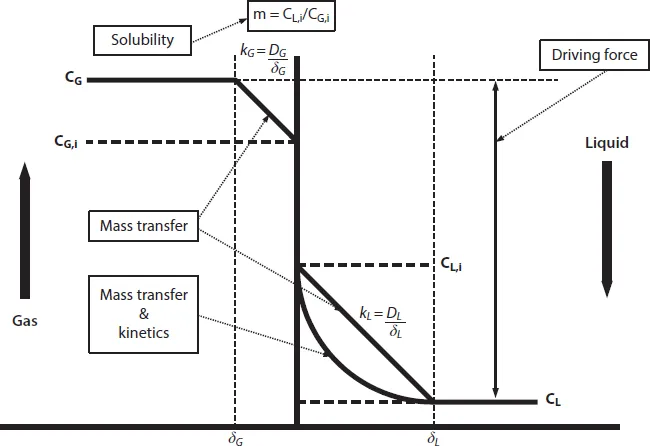
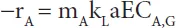
mA = physical solubility of component A in the solvent, -
kL = liquid side mass transfer coefficient, m.s−1
a = effective gas-liquid area, m2.m−3 reactor
E = chemical enhancement factor, -
CA,G = concentration of component A in gas phase, mol.m−3
Table of contents
- Cover
- Half Title page
- Title page
- Copyright page
- Preface
- Chapter 1: Rate-Base Simulations of Absorption Processes; Fata Morgana or Panacea?
- Chapter 2: Modelling in Acid Gas Removal Processes
- Chapter 3: Thermodynamic Approach of CO2 Capture, Combination of Experimental Study and Modeling
- Chapter 4: Employing Simulation Software for Optimized Carbon Capture Process
- Chapter 5: Expectations from Simulation
- Chapter 6: Calorimetry in Aqueous Solutions of Demixing Amines for Processes in CO2 Capture
- Chapter 7: Speciation in Liquid-Liquid Phase-Separating Solutions of Aqueous Amines for Carbon Capture Applications by Raman Spectroscopy
- Chapter 8: A Simple Model for the Calculation of Electrolyte Mixture Viscosities
- Chapter 9: Phase Equilibria Investigations of Acid Gas Hydrates: Experiments and Modelling
- Chapter 10: Thermophysical Properties, Hydrate and Phase Behaviour Modelling in Acid Gas-Rich Systems
- Chapter 11: “Self-Preservation” of Methane Hydrate in Pure Water and (Water +Diesel Oil + Surfactant) Dispersed Systems
- Chapter 12: The Development of Integrated Multiphase Flash Systems
- Chapter 13: Reliable PVT Calculations – Can Cubics Do It?
- Chapter 14: Vapor-Liquid Equilibria Predictions of Carbon Dioxide + Hydrogen Sulfide Mixtures Using the CPA, SRK, PR, SAFT, and PC-SAFT Equations of State
- Chapter 15: Capacity Control Considerations for Acid Gas Injection Systems
- Chapter 16: Review and Testing of Radial Simulations of Plume Expansion and Confirmation of Acid Gas Containment Associated with Acid Gas Injection in an Underpressured Clastic Carbonate Reservoir
- Chapter 17: Three-Dimensional Reservoir Simulation of Acid Gas Injection in Complex Geology – Process and Practice
- Chapter 18: Production Forecasting of Fractured Wells in Shale Gas Reservoirs with Discontinuous Micro-Fractures
- Chapter 19: Study on the Multi-Scale Nonlinear Seepage Flow Theory of Shale Gas Reservoir
- Chapter 20: CO2 EOR and Sequestration Technologies in PetroChina
- Chapter 21: Study on the Microscopic Residual Oil of CO2 Flooding for Extra-High Water-Cut Reservois
- Chapter 22: Monitoring of Carbon Dioxide Geological Utilization and Storage in China: A Review
- Chapter 23: Separation of Methane from Biogas by Absorption-Adsorption Hybrid Method
- Index