
Boron-Based Compounds
Potential and Emerging Applications in Medicine
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Boron-Based Compounds
Potential and Emerging Applications in Medicine
About this book
Noted experts review the current status of boron-containing drugs and materials for molecular medical diagnostics
Boron-Based Compounds offers a summary of the present status and promotes the further development of new boron-containing drugs and advanced materials, mostly boron clusters, for molecular medical diagnostics. The knowledge accumulated during the past decades on the chemistry and biology of bioorganic and organometallic boron compounds laid the foundation for the emergence of a new area of study and application of boron compounds as lipophilic pharmacophores and modulators of biologically active molecules.This important text brings together in one comprehensive volume contributions from renowned experts in the field of medicinal chemistry of boron compounds.
The authors cover a range of the most relevant topics including boron compounds as modulators of the bioactivity of biomolecules, boron clusters as pharmacophores or for drug delivery, boron compounds for boron neutron capture therapy (BNCT) and for diagnostics, as well as in silico molecular modeling of boron- and carborane-containing compounds in drug design. Authoritative and accessible, Boron-Based Compounds:
- Contains contributions from a panel of internationally renowned experts in the field
- Offers a concise summary of the current status of boron-containing drugs and materials used for molecular diagnostics
- Highlights the range and capacity of boron-based compounds in medical applications
- Includes information on boron neutron capture therapy and diagnostics
Designed for academic and industrial scientists, this important resource offers the cutting-edge information needed to understand the current state of boron-containing drugs and materials for molecular medical diagnostics.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
Part 1
Design of New Boron‐based Drugs
1.1
Carboranes as Hydrophobic Pharmacophores: Applications for Design of Nuclear Receptor Ligands
1.1.1 Roles of Hydrophobic Pharmacophores in Medicinal Drug Design
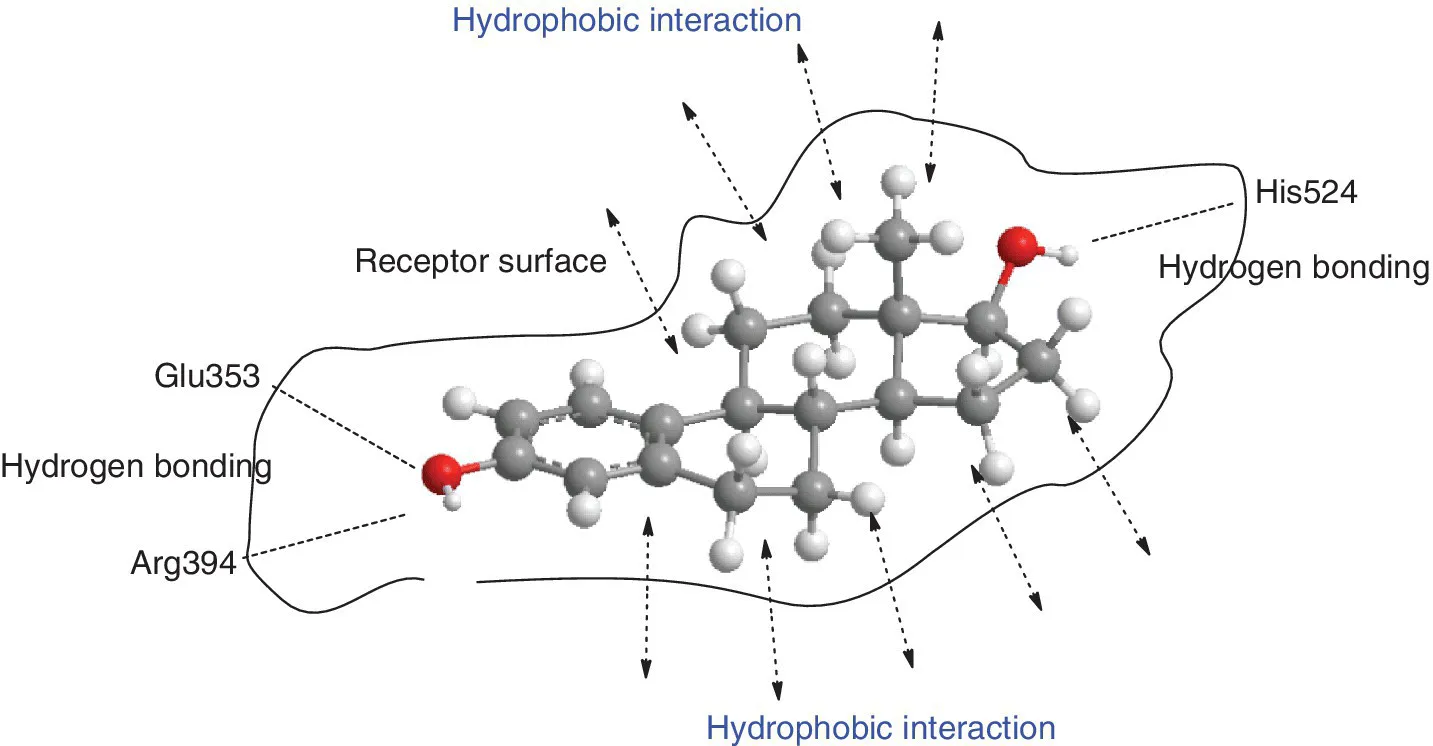
1.1.2 Carboranes as Hydrophobic Structures for Medicinal Drug Design
![Structures of dodecahedrane, fullerene C60, adamantane, bicyclo[2, 2, 2]octane, o-carborane, m-carborane, and p-carborane.](https://book-extracts.perlego.com/991769/images/c01f001_02-plgo-compressed.webp)
Table of contents
- Cover
- Title Page
- Table of Contents
- List of Contributors
- Preface
- Part 1: Design of New Boron‐based Drugs
- Part 2: Boron Compounds in Drug Delivery and Imaging
- Part 3: Boron Compounds for Boron Neutron Capture Therapy
- Index
- End User License Agreement