
Quantitative Pharmacology and Individualized Therapy Strategies in Development of Therapeutic Proteins for Immune-Mediated Inflammatory Diseases
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Quantitative Pharmacology and Individualized Therapy Strategies in Development of Therapeutic Proteins for Immune-Mediated Inflammatory Diseases
About this book
Thorough Overview Identifies and Addresses Critical Gaps in the Treatment of Several Chronic Diseases
With increasing numbers of patients suffering from Immune-Mediated Inflammatory Diseases (IMIDs), and with the increasing reliance on biopharmaceuticals to treat them, it is imperative that researchers and medical practitioners have a thorough understanding of the absorption, distribution, metabolism and excretion (ADME) of therapeutic proteins as well as translational pharmacokinetic/pharmacodynamic (PK/PD) modeling for them. This comprehensive volume answers that need to be addressed.
Featuring eighteen chapters from world-renowned experts and opinion leaders in pharmacology, translational medicine and immunology, editors Honghui Zhou and Diane Mould have curated a much-needed collection of research on the advanced applications of pharmacometrics and systems pharmacology to the development of biotherapeutics and individualized treatment strategies for the treatment of IMIDs. Authors discuss the pathophysiology of autoimmune diseases in addition to both theoretical and practical aspects of quantitative pharmacology for therapeutic proteins, current translational medicine research methodologies and novel thinking in treatment paradigm strategies for IMIDs. Other notable features include:
• Contributions from well-known authors representing leading academic research centers, specialized contract research organizations and pharmaceutical industries whose pipelines include therapeutic proteins
• Chapters on a wide range of topics (e.g., pathophysiology of autoimmune diseases, biomarkers in ulcerative colitis, model-based meta-analysis use in the development of therapeutic proteins)
• Case studies of applying quantitative pharmacology approaches to guiding therapeutic protein drug development in IMIDs such as psoriasis, inflammatory bowel disease, multiple sclerosis and lupus
Zhou and Mould's timely contribution to the critical study of biopharmaceuticals is a valuable resource for any academic and industry researcher working in pharmacokinetics, pharmacology, biochemistry, or biotechnology as well as the many clinicians seeking the safest and most effective treatments for patients dealing with chronic immune disorders.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
1
Disease Interception in Autoimmune Diseases: From a Conceptual Framework to Practical Implementation
1.1 Introduction to Disease Interception
1.1.1 What is Disease Interception and How Does This Impact Our Prospective Thinking Toward Novel Solutions for Patients Suffering from Autoimmune Diseases?
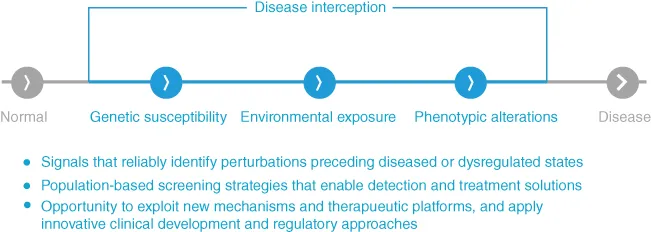
1.2 Disease Interception in Autoimmune Diseases
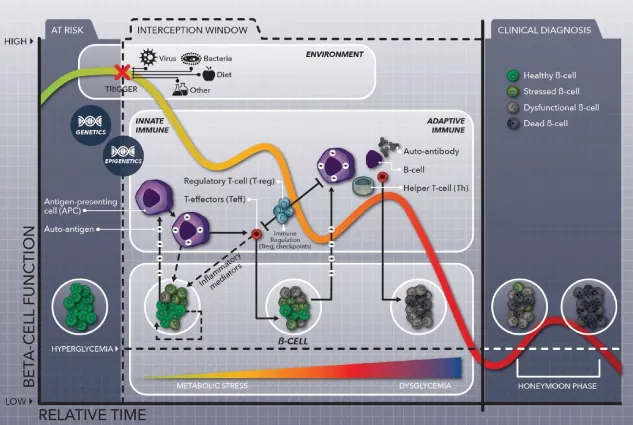
Table of contents
- Cover
- Table of Contents
- List of Contributors
- About the Editors
- Foreword
- Preface
- 1 Disease Interception in Autoimmune Diseases: From a Conceptual Framework to Practical Implementation
- 2 The Role of Biomarkers in Treatment Algorithms for Ulcerative Colitis (UC)
- 3 Mechanism and Physiologically Based PK/PD Model in Assisting Translation from Preclinical to Clinical: Understanding PK/PD of Therapeutic Proteins at Site‐of‐Action
- 4 Application of Minimal Anticipated Biological Effect Level (MABEL) in Human Starting Dose Selection for Immunomodulatory Protein Therapeutics – Principles and Case Studies
- 5 5Model‐Based Meta‐Analysis Use in the Development of Therapeutic Proteins
- 6 Utility of Joint Population Exposure–Response Modeling Approach to Assess Multiple Continuous and Categorical Endpoints in Immunology Drug Development
- 7 Modeling Approaches to Characterize Target‐Mediated Pharmacokinetics and Pharmacodynamics for Therapeutic Proteins
- 8 Tutorial: Numerical (NONMEM) Implementation of the Target‐Mediated Drug Disposition Model
- 9 Translational Considerations in Developing Bispecific Antibodies: What Can We Learn from Quantitative Pharmacology?
- 10 Application of Pharmacometrics and Systems Pharmacology to Current and Emerging Biologics in Inflammatory Bowel Diseases
- 11 Pharmacokinetics‐Based Dosing for Therapeutic Monoclonal Antibodies in Inflammatory Bowel Disease
- 12 Pharmacokinetics‐Based Dosing Strategies for Therapeutic Proteins in Inflammatory Bowel Disease
- 13 Quantitative Pharmacology Approach to Select Optimal Dose and Study the Important Factors in Determining Disposition of Therapeutic Monoclonal Antibody in Pediatric Subjects – Some Considerations
- 14 Quantitative Pharmacology Assessment Strategy Therapeutic Proteins in Pediatric Subjects – Challenges and Opportunities
- 15 Case Examples of Using Quantitative Pharmacology in Developing Therapeutic Proteins for Plaque Psoriasis – Guselkumab
- 16 Vedolizumab—A Case Example of Using Quantitative Pharmacology in Developing Therapeutic Biologics in Inflammatory Bowel Disease
- 17 Case Examples of Using Quantitative Pharmacology in Developing Therapeutic Proteins in Systemic Lupus Erythematosus – Belimumab
- 18 Case Examples of Using Quantitative Pharmacology in Developing Therapeutic Proteins in Multiple Sclerosis – Peginterferon Beta‐1a, Daclizumab Beta, Natalizumab
- Index
- End User License Agreement