
- English
- ePUB (mobile friendly)
- Available on iOS & Android
About this book
Provides students and practitioners with a comprehensive understanding of the theory of spectroscopy and the design and use of spectrophotometers
In this book, you will learn the fundamental principles underpinning molecular spectroscopy and the connections between those principles and the design of spectrophotometers.
Spectroscopy, along with chromatography, mass spectrometry, and electrochemistry, is an important and widely-used analytical technique. Applications of spectroscopy include air quality monitoring, compound identification, and the analysis of paintings and culturally important artifacts. This book introduces students to the fundamentals of molecular spectroscopy – including UV-visible, infrared, fluorescence, and Raman spectroscopy – in an approachable and comprehensive way. It goes beyond the basics of the subject and provides a detailed look at the interplay between theory and practice, making it ideal for courses in quantitative analysis, instrumental analysis, and biochemistry, as well as courses focused solely on spectroscopy. It is also a valuable resource for practitioners working in laboratories who regularly perform spectroscopic analyses.
Spectroscopy: Principles and Instrumentation:
- Provides extensive coverage of principles, instrumentation, and applications of molecular spectroscopy
- Facilitates a modular approach to teaching and learning about chemical instrumentation
- Helps students visualize the effects that electromagnetic radiation in different regions of the spectrum has on matter
- Connects the fundamental theory of the effects of electromagnetic radiation on matter to the design and use of spectrophotometers
- Features numerous figures and diagrams to facilitate learning
- Includes several worked examples and companion exercises throughout each chapter so that readers can check their understanding
- Offers numerous problems at the end of each chapter to allow readers to apply what they have learned
- Includes case studies that illustrate how spectroscopy is used in practice, including analyzing works of art, studying the kinetics of enzymatic reactions, detecting explosives, and determining the DNA sequence of the human genome
- Complements Chromatography: Principles and Instrumentation
The book is divided into five chapters that cover the Fundamentals of Spectroscopy, UV-visible Spectroscopy, Fluorescence/Luminescence Spectroscopy, Infrared Spectroscopy, and Raman Spectroscopy. Each chapter details the theory upon which the specific techniques are based, provides ways for readers to visualize the molecular-level effects of electromagnetic radiation on matter, describes the design and components of spectrophotometers, discusses applications of each type of spectroscopy, and includes case studies that illustrate specific applications of spectroscopy.
Each chapter is divided into multiple sections using headings and subheadings, making it easy for readers to work through the book and to find specific information relevant to their interests. Numerous figures, exercises, worked examples, and end-of-chapter problems reinforce important concepts and facilitate learning.
Spectroscopy: Principles and Instrumentation is an excellent text that prepares undergraduate students and practitioners to operate in modern laboratories.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1
FUNDAMENTALS OF SPECTROSCOPY
1.1. PROPERTIES OF ELECTROMAGNETIC RADIATION
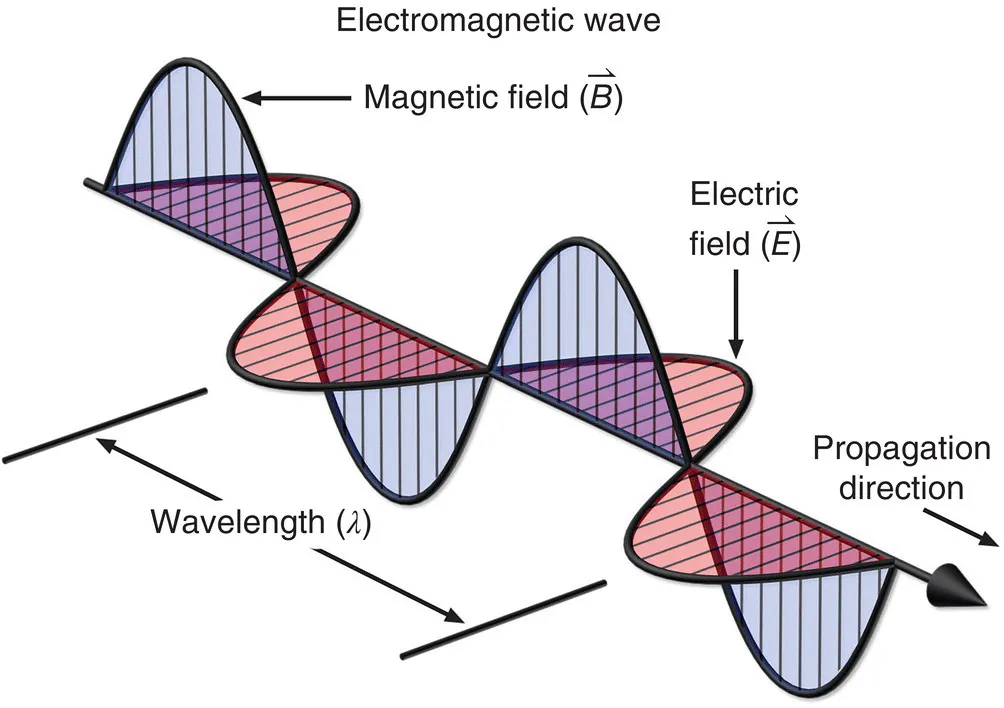
- Speed
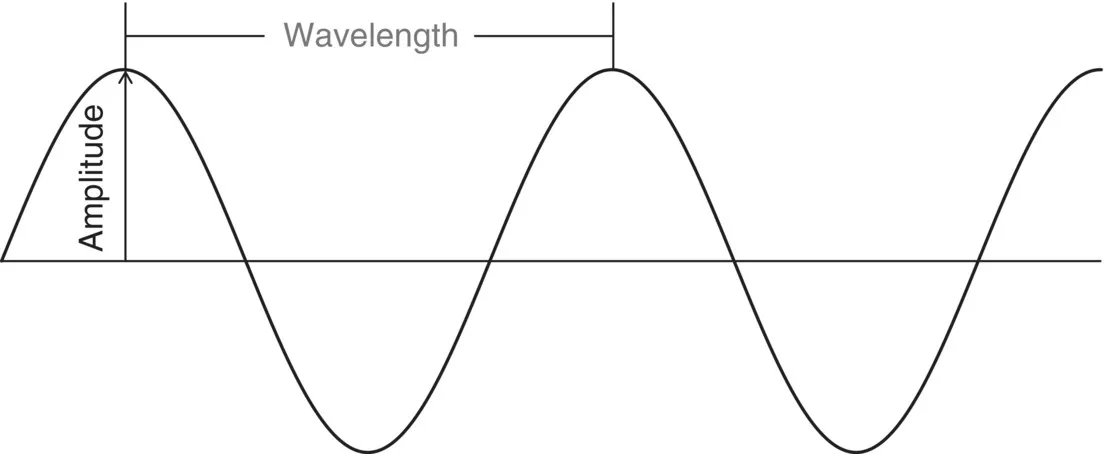
- Amplitude
- Frequency
- Wavelength
- Energy
1.1.1. Speed, c
1.1.2. Amplitude, A
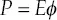
1.1.3. Frequency, υ
Table of contents
- COVER
- TABLE OF CONTENTS
- PREFACE
- 1 FUNDAMENTALS OF SPECTROSCOPY
- 2 UV‐VISIBLE SPECTROPHOTOMETRY
- 3 MOLECULAR LUMINESCENCE
- 4 INFRARED SPECTROSCOPY
- 5 RAMAN SPECTROSCOPY
- SOLUTIONS
- INDEX
- END USER LICENSE AGREEMENT
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app