
Materials and Processes for CO2 Capture, Conversion, and Sequestration
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Materials and Processes for CO2 Capture, Conversion, and Sequestration
About this book
Addresses materials, technology, and products that could help solve the global environmental crisis once commercialized
This multidisciplinary book encompasses state-of-the-art research on the topics of Carbon Capture and Storage (CCS), and complements existing CCS technique publications with the newest research and reviews. It discusses key challenges involved in the CCS materials design, processing, and modeling and provides in-depth coverage of solvent-based carbon capture, sorbent-based carbon capture, membrane-based carbon capture, novel carbon capture methods, computational modeling, carbon capture materials including metal organic frameworks (MOF), electrochemical capture and conversion, membranes and solvents, and geological sequestration.
Materials and Processes for CO 2 Capture, Conversion and Sequestration offers chapters on: Carbon Capture in Metal-Organic Frameworks; Metal Organic Frameworks Materials for Post-Combustion CO 2 Capture; New Progress of Microporous Metal-Organic Frameworks in CO 2 Capture and Separation; In Situ Diffraction Studies of Selected Metal-Organic Framework (MOF) Materials for Guest Capture Applications; Electrochemical CO 2 Capture and Conversion; Electrochemical Valorization of Carbon Dioxide in Molten Salts; Microstructural and Structural Characterization of Materials for CO 2 Storage using Multi-Scale X-Ray Scattering Methods; Contribution of Density Functional Theory to Microporous Materials for Carbon Capture; and Computational Modeling Study of MnO 2 Octahedral Molecular Sieves for Carbon Dioxide Capture Applications.
- Addresses one of the most pressing concerns of society—that of environmental damage caused by the greenhouse gases emitted as we use fossil fuels
- Covers cutting-edge capture technology with a focus on materials and technology rather than regulation and cost
- Highlights the common and novel CCS materials that are of greatest interest to industrial researchers
- Provides insight into CCS materials design, processing characterization, and computer modeling
Materials and Processes for CO 2 Capture, Conversion and Sequestration is ideal for materials scientists and engineers, energy scientists and engineers, inorganic chemists, environmental scientists, pollution control scientists, and carbon chemists.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1
CARBON CAPTURE IN METAL–ORGANIC FRAMEWORKS
1.1 INTRODUCTION
1.1.1 The Importance of Carbon Dioxide Capture
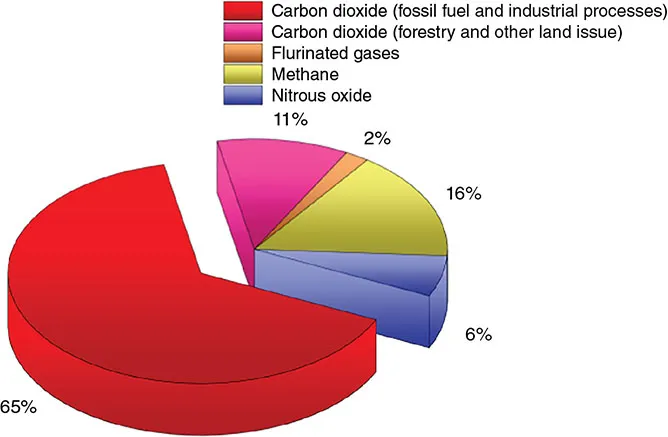
1.1.2 Conventional Industrial Process of Carbon Capture and Limitations: Liquid Amines
Table of contents
- Cover
- Title Page
- Copyright
- Preface
- List of Contributors
- 1 Carbon Capture in Metal–Organic Frameworks
- 2 Metal–Organic Frameworks Materials for Post-Combustion CO2 Capture
- 3 New Progress of Microporous Metal–Organic Frameworks in CO2 Capture and Separation
- 4 In Situ Diffraction Studies of Selected Metal–Organic Framework Materials for Guest Capture/Exchange Applications
- 5 Electrochemical CO2 Capture and Conversion
- 6 Electrochemical Valorization of Carbon Dioxide in Molten Salts
- 7 Microstructural and Structural Characterization of Materials for CO2 Storage Using Multi-Scale X-Ray Scattering Methods
- 8 Contribution of Density Functional Theory to Microporous Materials for Carbon Capture
- 9 Computational Modeling Study of MnO2 Octahedral Molecular Sieves for Carbon Dioxide–Capture Applications
- Index
- End User License Agreement
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app