
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
Textbook concisely introduces engineering thermodynamics, covering concepts including energy, entropy, equilibrium and reversibility
- Novel explanation of entropy and the second law of thermodynamics
- Presents abstract ideas in an easy to understand manner
- Includes solved examples and end of chapter problems
- Accompanied by a website hosting a solutions manual
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Energy, Entropy and Engines by Sanjeev Chandra in PDF and/or ePUB format, as well as other popular books in Technology & Engineering & Mechanical Engineering. We have over one million books available in our catalogue for you to explore.
Information
1
Introduction: A Brief Historyof Thermodynamics
In this chapter you will:
- Review the historical development of heat engines.
- Learn how thermodynamics grew out of efforts to improve the performance of heat engines.
- Gain an overview of concepts such as energy and entropy and the laws of thermodynamics.
1.1 What is Thermodynamics?
When earth’s creatures were created, according to Greek legends, each received its own gift of speed or strength or courage. Some animals received wings to soar on, others claws to defend themselves, but finally, when it was the turn of humans, nothing remained. Prometheus saved mankind by stealing fire from the gods, making people far more powerful than any animal. Such myths – and similar stories exist in almost every society – trace the birth of human civilisation to the discovery of fire, which gave warmth, nourishment and the ability to craft objects out of stone and metal.
Fire alone would not have allowed humans to survive in the wilderness – they also needed tools. Life without sharp claws or fangs is possible if you can make knives and spearheads. Humans may not have the speed of a gazelle but they discovered wheels; levers and pulleys can lift heavier loads than any elephant. Tools improved slowly over time as wheelbarrows evolved into horse drawn carts and stones for grinding grain became windmills, but there were few gains made in the power used to drive them. Animals, water and wind were all harnessed to drive machines, but there is a limit to how effective any of these power sources are. Winds are unreliable, there are a finite number of sites with running water, and only a few horses can be hitched to a cart at one time. This lack of power sources limited how fast technology could evolve over most of human history. An ancient Egyptian, transported 30 centuries forward to medieval Europe, would have had little difficulty in recognising the machines used.
Then, a little over 300 years ago, fire was used as a power source for the first time. The first practical steam engine marked a turning point in human history, for it put enormous reserves of energy at our disposal. We are no longer restricted to capturing forces exerted by the elements or animals. Gases expand when heated and exert tremendous pressures that can be exploited to drive power plants, aircraft and automobiles. The only constraint on generating power is the amount of heat available, and the technology used to generate heat has advanced rapidly, whether it is by burning fuel, capturing solar radiation, or splitting atoms in nuclear reactions. Today, machines that use heat to produce work are everywhere.
As steam engines became more common, questions about them multiplied. What is the relation between heat and work? How much work can be obtained if a given amount of fuel is burned? Can the performance of engines be improved? Thermodynamics was the science that grew from efforts to answer these questions. The word itself is a combination of the Greek therme, meaning heat, and dynamis, meaning force, and thermodynamics is often defined as the science that studies the relationship between heat and work. Engineers struggling to understand how engines work formulated the principles of thermodynamics, but they have since been used in the study of all phenomena that involve changes in energy. Astrophysicists use the laws of thermodynamics to predict the fate of an exploding star, biologists apply them to the metabolism of animals and chemists rely on them to determine the products of chemical reactions.
1.2 Steam Engines
Using heat to produce motion is not a very novel achievement. As early as the first century a Greek inventor had designed a toy in which steam escaping from nozzles mounted on the surface of a metal sphere made it spin, but there seems to have been no practical application of this device. In subsequent centuries cannons became the most impressive illustrations of how objects could be transported by generating heat. But, no matter how spectacular the discharge of a cannon, it is difficult to harness it for any constructive purpose. For that we need a “heat engine”, defined as a device that operates continuously, producing work as long as heat is supplied to it.
We can mark precisely the date when the first industrial heat engine was invented, for in 1698 the king of England was pleased to grant Thomas Savery a patent for a “fire engine” to be used “… for raising of water, and occasioning motion to all sorts of mill works …”. Savery’s machine did not resemble our typical image of a steam engine, for it had no furiously driving pistons or spinning flywheels. It consisted (see Figure 1.1) of a large chamber that was first filled with steam from a boiler, sealed and then sprayed with cold water to condense the steam in the vessel and create a partial vacuum that sucked water up from an underground mine. High-pressure steam was used to empty the chamber by pushing the water in it up to a higher level. Valves controlling the flow of steam and water were operated manually and a good operator could complete several cycles in a minute.
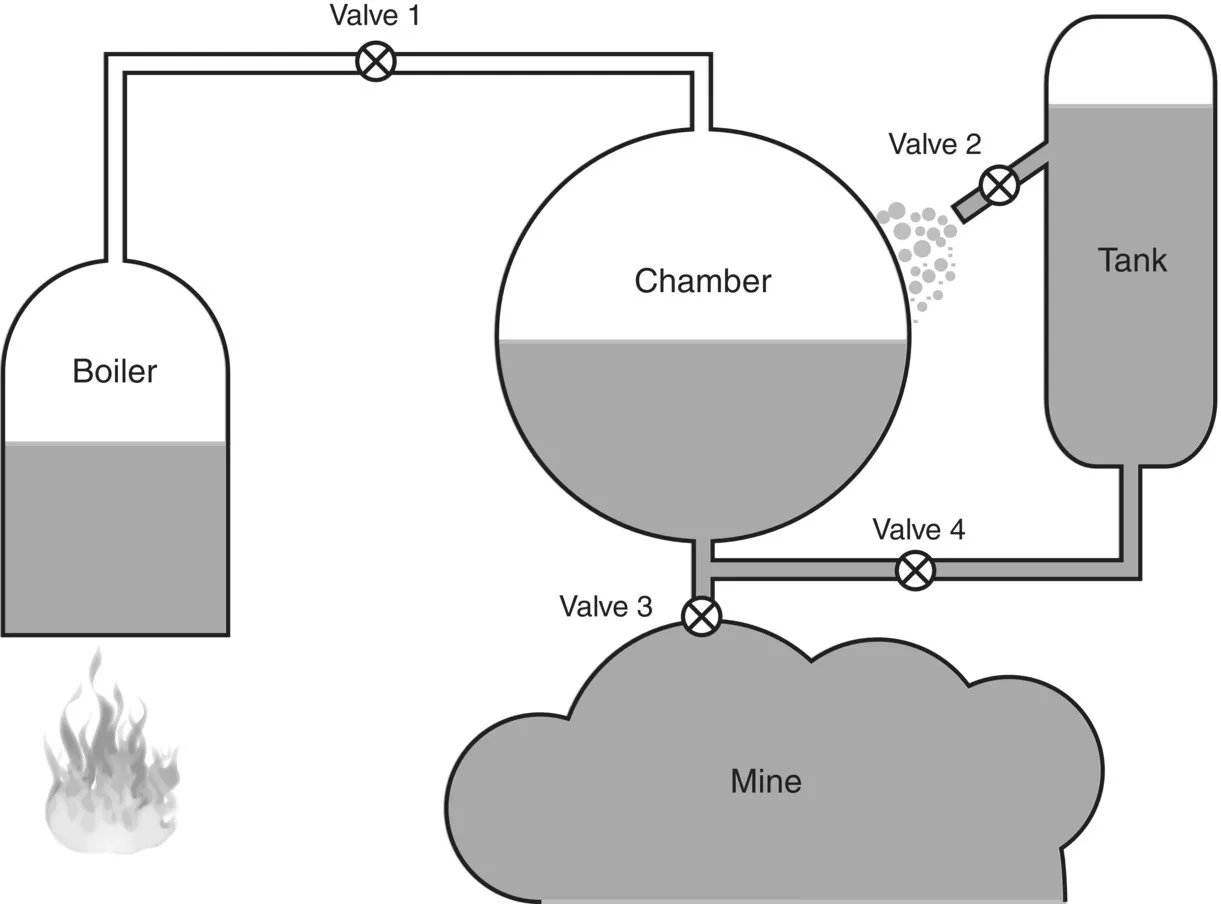
Figure 1.1 Savery Engine. Opening valve 1 fills the chamber with steam. Opening valve 2 douses the chamber with cold water, condensing the steam and creating a partial vacuum. Opening valve 3 sucks water into the evacuated chamber from the flooded mine. Opening valve 4 while filling the chamber with steam pushes water into the tank.
Savery intended to sell his pumps to English coal mines where flooding was a frequent occurrence, so that water had to be drained by hand or horse driven pumps. Sadly, his pumps proved to be rather leaky so that it was hard to hold a very good vacuum in the chamber. Savery claimed that his engine could raise water by about 80 feet (24.4 m), which would have required steam pressures of almost three atmospheres. Frequent explosions of poorly made boilers were so common that high-pressure steam was viewed with fear and not used again for more than a century when manufacturing techniques had greatly improved.
Savery was unable to produce a commercially successful engine, but he proved that steam could be used to drive machines. By the time Savery’s 14-year patent expired in 1712 Thomas Newcomen was ready with his design for a new engine, which looks far more recognisable to us as a steam engine (Figure 1.2). Newcomen had a piston moving back and forth in a cylindrical chamber, one side of which was connected to a boiler producing steam. When the chamber was filled with steam the piston rose up. Spraying water into the chamber condensed the steam, producing a partial vacuum so that atmospheric pressure forced the piston down. A beam connected to the piston oscillated as the piston moved up and down, which could be used to drive a pump or some other machine. This was an “atmospheric engine”, in which work was done by the atmosphere pushing a piston against a vacuum. Steam pressure was never much higher than one atmosphere, minimising the hazard of explosions.
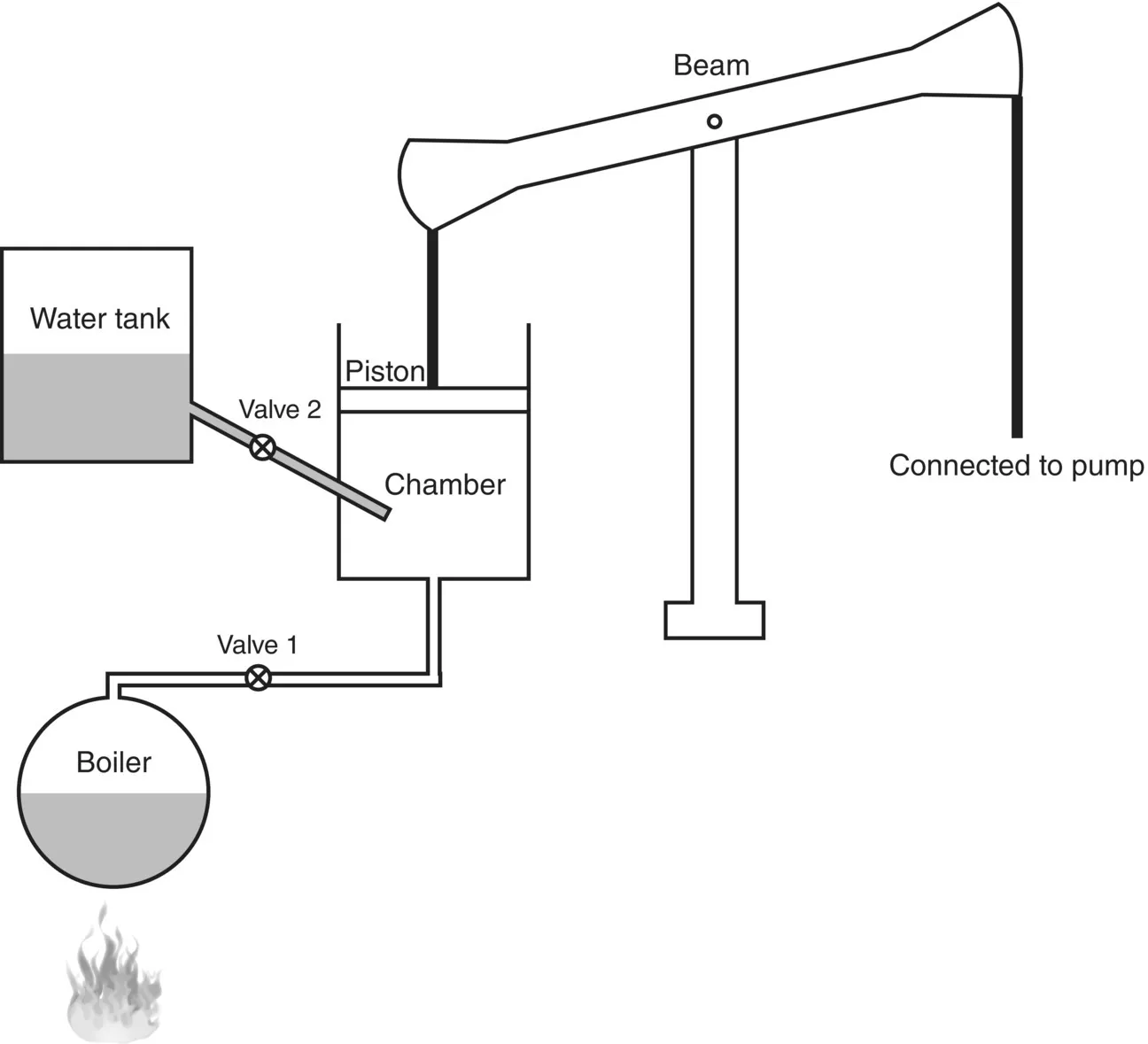
Figure 1.2 Newcomen Engine. Opening valve 1 sends steam into the chamber and raises the piston. Opening valve 2 sprays water into the chamber, condensing the steam so that atmospheric pressure forces the piston down. A beam connected to the piston oscillates up and down and drives the pump.
Newcomen’s engine was an immense success and hundreds were built and sold. They were initially used to power pumps in coalmines but soon found new applications in textile mills and other factories. For over 50 years Newcomen’s engines represented the most sophisticated technology available and sparked a remarkable technical and social transformation that changed human history. For the first time machines could work non-stop without depending on beasts of burden or being subject to the vagaries of weather. Any factory had access to as much power as it needed, no matter where it was located. The steam engine gave birth to the industrial revolution and created the modern world.
Newcomen’s engines were a tremendous accomplishment but consumed enormous amounts of coal to generate steam, most of which was wasted. At the start of each cycle, when steam entered the cylinder, much of its energy went into heating the walls of the cylinder, only to have to cool them down again when the steam was condensed with a water spray. While engines were confined to coalmines this was not of great concern since fuel was practically free, but when they began to be used in factories far from fuel supplies operating costs became a serious problem.
James Watt, a young instrument maker at the University of Glasgow, proposed a solution. Watt had been assigned the job of fixing a model of a Newcomen engine used for laboratory demonstrations. He found that the model worked as designed, but consumed all the steam supplied simply to heat the cylinder wall. After much thought he solved the problem by adding an external chamber in which steam was condensed (Figure 1.3). In 1769 Watt obtained a patent for the external condenser, entitled a “new method for lessening the consumption of steam and fuel in fire engines”. The new engines were so much more efficient than the older Newcomen engines that it became feasible to use them in many new industrial applications.
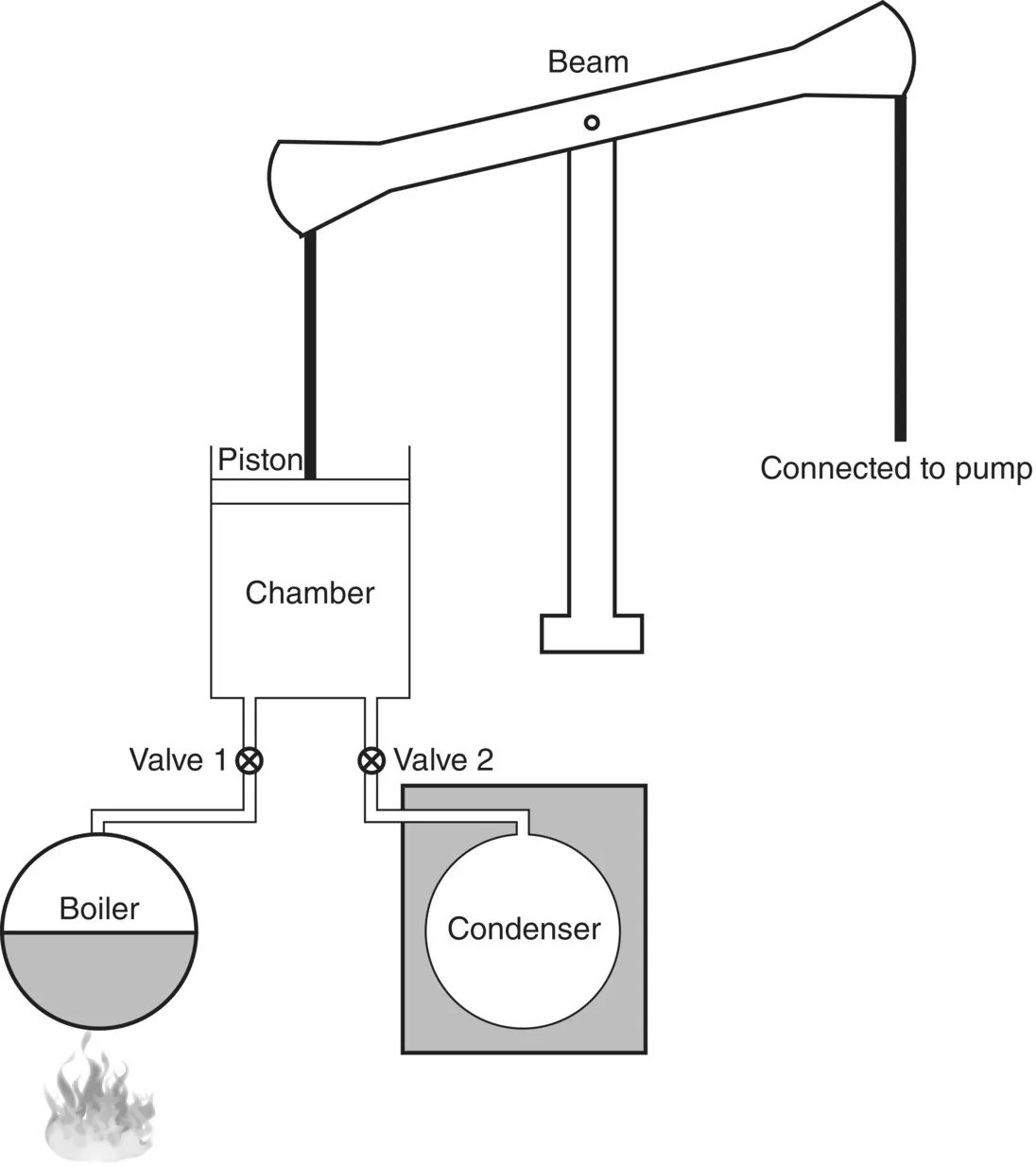
Fig...
Table of contents
- Cover
- Title Page
- Table of Contents
- Preface
- About the Companion Website
- 1 Introduction
- 2 Concepts and Definitions
- 3 Thermodynamic System Properties
- 4 Energy and the First Law of Thermodynamics
- 5 Entropy
- 6 The Second Law of Thermodynamics
- 7 Phase Equilibrium
- 8 Ideal Heat Engines and Refrigerators
- 9 Vapour Power and Refrigeration Cycles
- 10 Gas Power Cycles
- Appendices
- Index
- End User License Agreement