
Chemistry of the Carbonyl Group
A Step-by-Step Approach to Understanding Organic Reaction Mechanisms
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Chemistry of the Carbonyl Group
A Step-by-Step Approach to Understanding Organic Reaction Mechanisms
About this book
Teaches and enables students to build confidence in drawing and manipulating curly arrows, a fundamental skill for all organic chemists
This book is an interactive approach to learning about chemistry of the carbonyl group—inviting students to work through its pages with pencil and paper in hand. It educates with the belief that the most effective way to learn is by practice and interaction. With this in mind, the reader is asked to predict what would happen under a specific set of reaction conditions. The book is divided into frames: each frame poses a question and invites the reader to predict what will happen. Subsequent frames give the solution but then pose more questions to develop a theme further.
Chemistry of the Carbonyl Group: A Programmed Approach to Organic Reaction Mechanisms, Revised Edition provides a solid grounding in the fundamental reactions of carbonyls. Presented in full colour to enhance the understanding of mechanisms within chemistry, the chapters of this step-by-step guide cover: nucleophilic addition to the carbonyl group; nucleophilic substitution; nucleophilic substitution at the carbonyl group with complete removal of carbonyl oxygen; carbanions and enolisation; and building organic molecules from carbonyl compounds.
- A must-have book for undergraduate chemists to emphasise understanding in carbonyl group chemistry
- Goes through all the stages of basic carbonyl chemistry, detailing even the simplest mechanisms
- A step-by-step learning guide to synthetic chemistry for the first year of a chemistry degree, with all the information needed for independent learning
- Provides a solid grounding in the fundamental reactions of carbonyls which will inform the understanding of many other organic chemistry reactions
Chemistry of the Carbonyl Group: A Programmed Approach to Organic Reaction Mechanisms - Revised Edition is packed with all the information on synthetic chemistry that every first-year student will need in order to learn independently.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
Chapter 1
Nucleophilic Addition to the Carbonyl Group
- ▪ - and
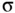 -bonds.
-bonds.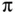
- ▪ Polarisable bonds.
- ▪ Electrophile and nucleophile.
- ▪ Conjugation with -bonds and lone pairs.
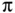
- ▪ Inductive effects.
- ▪ values.
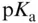
- ▪ Periodic table.
- ▪ Transition states.
- ▪ Acid catalysis.
- ▪ Instability of R2C(OR)2 in acid solution. Driving equilibria in a chosen direction by the use of acid, solvent, etc.
- ▪ Stability of different carbonyl compounds. Stability and reactivity as two sides of the same coin.
- ▪ Effect of substituents on equilibria. Relationship between basicity and nucleophilicity.
- ▪ Organo-magnesium compounds as nucleophiles.
- ▪ Use of Grignard reagents in syntheses.
- ▪ Ease of dehydration of tertiary alcohols in acid solution.
- ▪ Sources of nucleophilic H−.
- ▪ Use of Al and B compounds and as anion transferring reagents.
- ▪ Drawing transition states.
- ▪ Stability of the six-membered ring.
- ▪ Use of protecting groups.
- ▪ Use of reaction mechanisms in syntheses.
- 1. The carbonyl group (1a) has an easily polarisable -bond with an electrophilic carbon atom at one end easily attacked by nucleophiles:
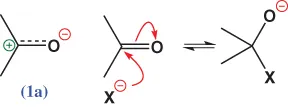 Write down the reaction (with curly arrows) between acetone and hydroxide ion.
Write down the reaction (with curly arrows) between acetone and hydroxide ion. - 2. Have you actually written down the formulae of the reagents and drawn the arrows? The program won't be of much help to you unless you do.
- 3.
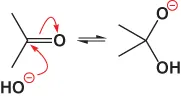 A nucleophile such as water uses its lone pair electrons (:) to attack and forms a neutral addition compound by proton transfer:
A nucleophile such as water uses its lone pair electrons (:) to attack and forms a neutral addition compound by proton transfer: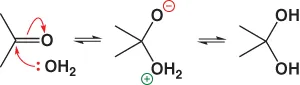 Note that only one proton is needed.Write down the reaction between the carbonyl compound acetaldehyde and the proton-bearing nucleophile ethanol.
Note that only one proton is needed.Write down the reaction between the carbonyl compound acetaldehyde and the proton-bearing nucleophile ethanol. - 4. If you find ...
Table of contents
- Cover
- Title Page
- Copyright
- Table of Contents
- Dedication
- Preface
- Acknowledgements
- Some Help That you May Need
- What Do You Need To Know Before You Start?
- Introduction
- Chapter 1: Nucleophilic Addition to the Carbonyl Group
- Chapter 2: Nucleophilic Substitution
- Chapter 3: Nucleophilic Substitution at the Carbonyl Group With Complete Removal of Carbonyl Oxygen
- Chapter 4: Carbanions and Enolisation
- Chapter 5: Building Organic Molecules From Carbonyl Compounds
- Index
- End User License Agreement