
eBook - ePub
Polymorphism in the Pharmaceutical Industry
Solid Form and Drug Development
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Polymorphism in the Pharmaceutical Industry
Solid Form and Drug Development
About this book
"Polymorphism in the Pharmaceutical Industry - Solid Form and Drug Development" highlights the relevance of polymorphism in modern pharmaceutical chemistry, with a focus on quality by design (QbD) concepts. It covers all important issues by way of case studies, ranging from properties and crystallization, via thermodynamics, analytics and theoretical modelling right up to patent issues.
As such, the book underscores the importance of solid-state chemistry within chemical and pharmaceutical development. It emphasizes why solid-state issues are important, the approaches needed to avoid problems and the opportunities offered by solid-state properties. The authors include true polymorphs as well as solvates and hydrates, while providing information on physicochemical properties, crystallization thermodynamics, quantum-mechanical modelling, and up-scaling. Important analytical tools to characterize solid-state forms and to quantify mixtures are summarized, and case studies on solid-state development processes in industry are also provided.
Written by acknowledged experts in the field, this is a high-quality reference for researchers, project managers and quality assurance managers in pharmaceutical, agrochemical and fine chemical companies as well as for academics and newcomers to organic solid-state chemistry.
As such, the book underscores the importance of solid-state chemistry within chemical and pharmaceutical development. It emphasizes why solid-state issues are important, the approaches needed to avoid problems and the opportunities offered by solid-state properties. The authors include true polymorphs as well as solvates and hydrates, while providing information on physicochemical properties, crystallization thermodynamics, quantum-mechanical modelling, and up-scaling. Important analytical tools to characterize solid-state forms and to quantify mixtures are summarized, and case studies on solid-state development processes in industry are also provided.
Written by acknowledged experts in the field, this is a high-quality reference for researchers, project managers and quality assurance managers in pharmaceutical, agrochemical and fine chemical companies as well as for academics and newcomers to organic solid-state chemistry.
Trusted by 375,005 students
Access to over 1 million titles for a fair monthly price.
Study more efficiently using our study tools.
Information
1
Solid State and Polymorphism of the Drug Substance in the Context of Quality by Design and ICH Guidelines Q8–Q12
Markus von Raumer1 and Rolf Hilfiker2
1 Idorsia Pharmaceuticals Ltd., Hegenheimermattweg 91, Allschwil, 4123, Switzerland
2 Solvias AG, Römerpark 2, Kaiseraugst, 4303, Switzerland
1.1 Introduction
The way in which the pharmaceutical industry is approaching technical development has evolved very much in the recent years. Fresh concepts coming from other industries have been introduced with the desire to push for a more science and risk‐based development approach throughout the product life cycle. Quality by design (QbD) in the pharmaceutical industry is an outcome of the efforts to harmonize development quality concepts and understandings by regulatory agencies and resulted in the International Conference of Harmonization (ICH) guidelines Q8 [1], Q9 [2], Q10 [3], Q11 [4], and Q12 [5]. Although first devised for pharmaceutical development (Q8), the QbD concepts and related tools were rapidly recognized as being very helpful for chemical development. A result of this process was the Q11 guideline that provides guidance for drug substance as defined in the scope of the ICH guideline Q6A [6] (this guideline contains the well‐known decision trees for polymorphism).
The scope of this chapter is to give a short introduction to the solid‐state development process in the pharmaceutical industry and to QbD. Questions on how QbD principles can be applied to solid‐state development will be discussed, highlighting how the solid state is an important parameter to be considered in the pharmaceutical development process. For that purpose, some general insights into the relevance of the drug substance (DS) solid state throughout various fields of pharmaceutical development will be given.
1.2 A Short Introduction to Polymorphism and Solid‐State Development
Only a brief overview of solid‐state development and polymorphism shall be given here. Subsequent chapters in this book will discuss the various aspects in more detail.
Many organic and inorganic compounds can exist in different solid forms [7–12]. They can be in the amorphous (Chapter ), i.e. disordered [13], or in the crystalline, i.e. ordered, state. In accordance with McCrone's definition [8], “The polymorphism of any element or compound is its ability to crystallize as more than one distinct crystal species,” we will call different crystal arrangements of the same chemical composition polymorphs (Figure 1.1). Especially in the pharmaceutical context, the term “polymorph or polymorphism” is used more broadly by many authors and regulatory agencies. The amorphous state, as well as hydrates or solvates (both of which do not have the same chemical composition), are tacitly included by the term. Because different inter‐ and intramolecular interactions such as van der Waals interactions and hydrogen bonds will be present in different crystal structures, different polymorphs will have different free energies and therefore different physical properties such as solubility, chemical stability, melting point, density, etc. (Chapter ). Hence, the crystal form of a solid material in development is often considered a critical quality attribute (CQA, see next section). Of practical importance are also solvates [14], sometimes called pseudopolymorphs, where solvent molecules are incorporated in the crystal lattice in a stoichiometric or nonstoichiometric [12, 15] way. Hydrates (Chapter ), where the solvent is water, are of particular interest because of the omnipresence of water. In addition to the crystalline, amorphous, and liquid states, condensed matter can exist in various mesophases. These mesophases are characterized by exhibiting partial order between that of a crystalline and an amorphous state [16, 17]. Several drug substances are known to form liquid crystalline phases, which can be either thermotropic, where the liquid crystal formation is induced by temperature, or lyotropic, where the transition is solvent induced [18–20].
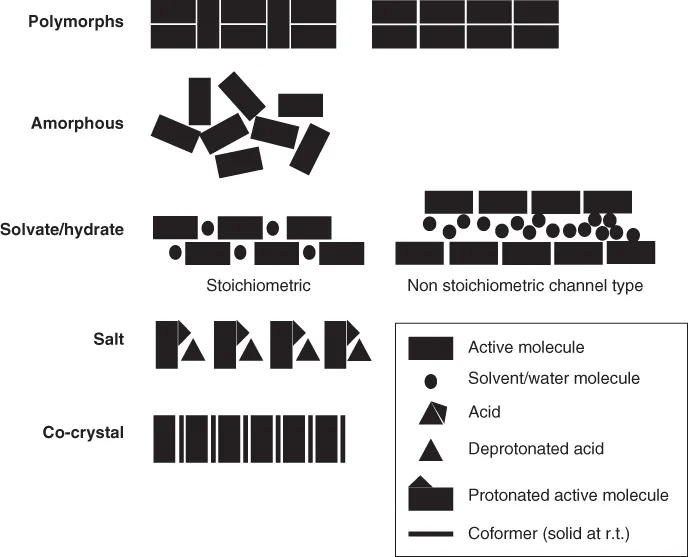
Figure 1.1 Schematic depiction of various types of solid forms.
Polymorphism is a very common phenomenon [11, 21–25] in connection with small‐molecule drug substances. The literature values concerning the prevalence of true polymorphs range from 32% [26] to 51% [27–29] of small organic molecules (molecular weight <600 g mol–1). According to the same references, 56% and 87%, respectively, have more than one solid form if solvates are included in the count.
In the context of pharmaceutical solid‐state development, polymorph considerations are made subsequent to general considerations like salt [30] (Chapter ) or co‐crystal [31] (Chapter ) formation. When a compound is acidic or basic, it is often possible to create a salt with a suitable base or acid, and such a salt can, in turn, often be crystallized. Crystalline salts may then again be able to exist as various polymorphs or solvates. From the scientific perspective, solvates can be considered as co‐crystals of the active molecule and solvent. In the pharmaceutical industry, the term co‐crystal is used in a slightly different way, however. A pharmaceutical co‐crystal is a solid, where the constituting molecules are in the solid phase as single components at room temperature. Obviously, solvates, co‐crystals and salts will have different properties than the polymorphs of the active molecule. About half of all active molecules are marketed as salts [25, 30, 32]. Recently, also the first co‐crystal composed of two active molecules reached the market (Entresto from Novartis [33]). Polymorphs, solvates, salts, and co‐crystals are schematically depicted in Figure 1.1. We will use the term “drug substance” for the therapeutic moiety, which may be a solvate, salt, or a co‐crystal, whereas the single, uncharged molecule will be called the “active molecule.”
1.3 A Short Introduction to Quality by Design (QbD)
Only a brief overview of QbD shall be given here. Pharmaceutical applications thereof were describ...
Table of contents
- Cover
- Table of Contents
- Preface to the Second Edition
- 1 Solid State and Polymorphism of the Drug Substance in the Context of Quality by Design and ICH Guidelines Q8–Q12
- 2 Alternative Solid Forms: Salts
- 3 Alternative Solid Forms: Co‐crystals
- 4 Thermodynamics of Polymorphs and Solvates
- 5 Toward Computational Polymorph Prediction
- 6 Hygroscopicity and Hydrates in Pharmaceutical Solids
- 7 The Amorphous State
- 8 Approaches to Solid‐Form Screening
- 9 Nucleation
- 10 Crystallization Process Modeling
- 11 Crystallization Process Scale‐Up, a Quality by Design (QbD) Perspective
- 12 Processing‐Induced Phase Transformations and Their Implications on Pharmaceutical Product Quality
- 13 Surface and Mechanical Properties of Molecular Crystals
- 14 Analytical Tools to Characterize Solid Forms
- 15 Industry Case Studies
- 16 Pharmaceutical Crystal Forms and Crystal‐Form Patents: Novelty and Obviousness1
- Index
- End User License Agreement
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Polymorphism in the Pharmaceutical Industry by Rolf Hilfiker, Markus von Raumer, Rolf Hilfiker,Markus von Raumer in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Industrial & Technical Chemistry. We have over one million books available in our catalogue for you to explore.