
- English
- ePUB (mobile friendly)
- Available on iOS & Android
About this book
Understanding, identifying and influencing the biological systems are the primary objectives of
chemical biology. From this perspective, metal complexes have always been of great assistance
to chemical biologists, for example, in structural identification and purification of essential
biomolecules, for visualizing cellular organelles or to inhibit specific enzymes. This inorganic side
of chemical biology, which continues to receive considerable attention, is referred to as inorganic
chemical biology.
Inorganic Chemical Biology: Principles, Techniques and Applications provides a comprehensive
overview of the current and emerging role of metal complexes in chemical biology. Throughout all
of the chapters there is a strong emphasis on fundamental theoretical chemistry and experiments
that have been carried out in living cells or organisms. Outlooks for the future applications of
metal complexes in chemical biology are also discussed.
Topics covered include:
• Metal complexes as tools for structural biology
• IMAC, AAS, XRF and MS as detection techniques for metals in chemical biology
• Cell and organism imaging and probing DNA using metal and metal carbonyl complexes
• Detection of metal ions, anions and small molecules using metal complexes
• Photo-release of metal ions in living cells
• Metal complexes as enzyme inhibitors and catalysts in living cells
Written by a team of international experts, Inorganic Chemical Biology: Principles, Techniques and
Applications is a must-have for bioinorganic, bioorganometallic and medicinal chemists as well as
chemical biologists working in both academia and industry.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
Chapter 1
New Applications of Immobilized Metal Ion Affinity Chromatography in Chemical Biology
1.1 Introduction
1.2 Principles and Traditional Use
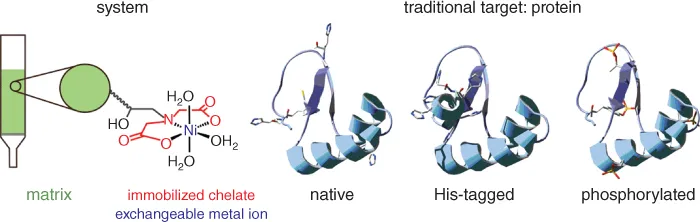
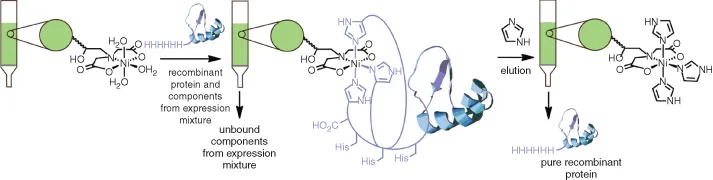
Table of contents
- Cover
- Title Page
- Copyright
- About the Editor
- List of Contributors
- Preface
- References
- Acknowledgements
- Chapter 1: New Applications of Immobilized Metal Ion Affinity Chromatography in Chemical Biology
- Chapter 2: Metal Complexes as Tools for Structural Biology
- Chapter 3: AAS, XRF, and MS Methods in Chemical Biology of Metal Complexes
- Chapter 4: Metal Complexes for Cell and Organism Imaging
- Chapter 5: Cellular Imaging with Metal Carbonyl Complexes
- Chapter 6: Probing DNA Using Metal Complexes
- Chapter 7: Visualization of Proteins and Cells Using Dithiol-reactive Metal Complexes
- Chapter 8: Detection of Metal Ions, Anions and Small Molecules Using Metal Complexes
- Chapter 9: Photo-release of Metal Ions in Living Cells
- Chapter 10: Release of Bioactive Molecules Using Metal Complexes
- Chapter 11: Metal Complexes as Enzyme Inhibitors and Catalysts in Living Cells
- Chapter 12: Other Applications of Metal Complexes in Chemical Biology
- Index
- End User License Agreement