
Ligand Design in Metal Chemistry
Reactivity and Catalysis
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Ligand Design in Metal Chemistry
Reactivity and Catalysis
About this book
The design of ancillary ligands used to modify the structural and reactivity properties of metal complexes has evolved into a rapidly expanding sub-discipline in inorganic and organometallic chemistry. Ancillary ligand design has figured directly in the discovery of new bonding motifs and stoichiometric reactivity, as well as in the development of new catalytic protocols that have had widespread positive impact on chemical synthesis on benchtop and industrial scales.
Ligand Design in Metal Chemistry presents a collection of cutting-edge contributions from leaders in the field of ligand design, encompassing a broad spectrum of ancillary ligand classes and reactivity applications. Topics covered include:
- Key concepts in ligand design
- Redox non-innocent ligands
- Ligands for selective alkene metathesis
- Ligands in cross-coupling
- Ligand design in polymerization
- Ligand design in modern lanthanide chemistry
- Cooperative metal-ligand reactivity
- P,N Ligands for enantioselective hydrogenation
- Spiro-cyclic ligands in asymmetric catalysis
This book will be a valuable reference for academic researchers and industry practitioners working in the field of ligand design, as well as those who work in the many areas in which the impact of ancillary ligand design has proven significant, for example synthetic organic chemistry, catalysis, medicinal chemistry, polymer science and materials chemistry.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
1
Key Concepts in Ligand Design: An Introduction
1.1 Introduction
1.2 Covalent bond classification and elementary bonding concepts
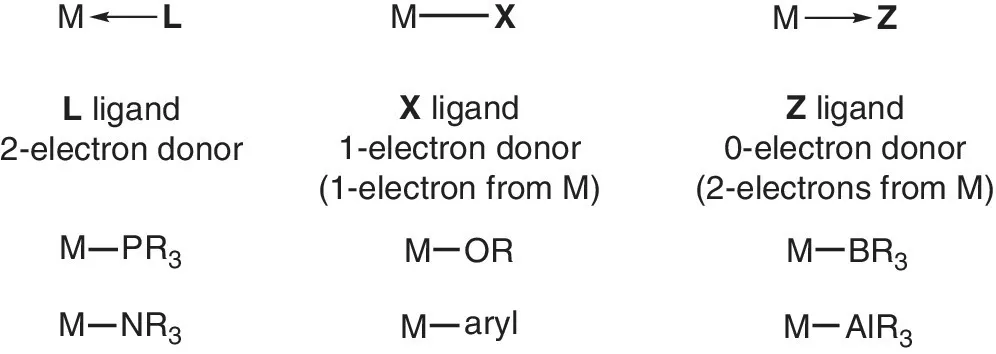
Table of contents
- Cover
- Title Page
- Table of Contents
- List of Contributors
- Foreword: Stephen L. Buchwald
- Foreword: David Milstein
- Preface
- 1 Key Concepts in Ligand Design: An Introduction
- 2 Catalyst Structure and Cis–Trans Selectivity in Ruthenium‐based Olefin Metathesis
- 3 Ligands for Iridium‐catalyzed Asymmetric Hydrogenation of Challenging Substrates
- 4 Spiro Ligands for Asymmetric Catalysis
- 5 Application of Sterically Demanding Phosphine Ligands in Palladium‐Catalyzed Cross‐Coupling leading to C(sp2)─E Bond Formation (E = NH2, OH, and F)
- 6 Pd‐N‐Heterocyclic Carbene Complexes in Cross‐Coupling Applications
- 7 Redox Non‐innocent Ligands: Reactivity and Catalysis
- 8 Ligands for Iron‐based Homogeneous Catalysts for the Asymmetric Hydrogenation of Ketones and Imines
- 9 Ambiphilic Ligands: Unusual Coordination and Reactivity Arising from Lewis Acid Moieties
- 10 Ligand Design in Enantioselective Ring‐opening Polymerization of Lactide
- 11 Modern Applications of Trispyrazolylborate Ligands in Coinage Metal Catalysis
- 12 Ligand Design in Modern Lanthanide Chemistry
- 13 Tight Bite Angle N,O‐Chelates. Amidates, Ureates and Beyond
- Index
- End User License Agreement