
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
MRI in Practice
About this book
MRI in Practice continues to be the number one reference book and study guide for the registry review examination for MRI offered by the American Registry for Radiologic Technologists (ARRT). This latest edition offers in-depth chapters covering all core areas, including: basic principles, image weighting and contrast, spin and gradient echo pulse sequences, spatial encoding, k-space, protocol optimization, artefacts, instrumentation, and MRI safety.
- The leading MRI reference book and study guide.
- Now with a greater focus on the physics behind MRI.
- Offers, for the first time, equations and their explanations and scan tips.
- Brand new chapters on MRI equipment, vascular imaging and safety.
- Presented in full color, with additional illustrations and high-quality MRI images to aid understanding.
- Includes refined, updated and expanded content throughout, along with more learning tips and practical applications.
- Features a new glossary.
MRI in Practice is an important text for radiographers, technologists, radiology residents, radiologists, and other students and professionals working within imaging, including medical physicists and nurses.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1
Basic principles
- Introduction
- Atomic structure
- Motion in the atom
- MR-active nuclei
- The hydrogen nucleus
- Alignment
- Net magnetic vector (NMV)
- Precession and precessional (Larmor) frequency
- Precessional phase
- Resonance
- MR signal
- The free induction decay (FID) signal
- Pulse timing parameters
After reading this chapter, you will be able to:
- Describe the structure of the atom.
- Explain the mechanisms of alignment and precession.
- Understand the concept of resonance and signal generation.
INTRODUCTION
The basic principles of magnetic resonance imaging (MRI) form the foundation for further understanding of this complex subject. It is important to grasp these ideas before moving on to more complicated topics in this book.
There are essentially two ways of explaining the fundamentals of MRI: classically and via quantum mechanics. Classical theory (accredited to Sir Isaac Newton and often called Newtonian theory) provides a mechanical view of how the universe (and therefore how MRI) works. Using classical theory, MRI is explained using the concepts of mass, spin, and angular momentum on a large or bulk scale. Quantum theory (accredited to several individuals including Max Planck, Albert Einstein, and Paul Dirac) operates at a much smaller, subatomic scale and refers to the energy levels of protons, neutrons, and electrons. Although classical theory is often used to describe physical principles on a large scale and quantum theory on a subatomic level, there is evidence that all physical principles are explained using quantum concepts [1]. However, for our purposes, this chapter mainly relies on classical perspectives because they are generally easier to understand. Quantum theory is only used to provide more detail when required.
In this chapter, we explore the properties of atoms and their interactions with magnetic fields as well as the mechanisms of excitation and relaxation.
ATOMIC STRUCTURE
All things are made of atoms. Atoms are organized into molecules, which are two or more atoms arranged together. The most abundant atom in the human body is hydrogen, but there are other elements such as oxygen, carbon, and nitrogen. Hydrogen is most commonly found in molecules of water (where two hydrogen atoms are arranged with one oxygen atom; H2O) and fat (where hydrogen atoms are arranged with carbon and oxygen atoms; the number of each depends on the type of fat).
The atom consists of a central nucleus and orbiting electrons (Figure 1.1). The nucleus is very small, one millionth of a billionth of the total volume of an atom, but it contains all the atom’s mass. This mass comes mainly from particles called nucleons, which are subdivided into protons and neutrons. Atoms are characterized in two ways.
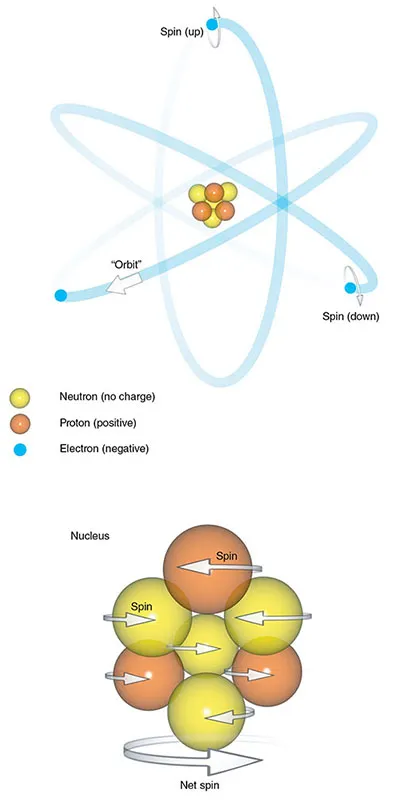
Figure 1.1 The atom.
- The atomic number is the sum of the protons in the nucleus. This number gives an atom its chemical identity.
- The mass number or atomic weight is the sum of the protons and neutrons in the nucleus.
The number of neutrons and protons in a nucleus is usually balanced so that the mass number is an even number. In some atoms, however, there are slightly more or fewer neutrons than protons. Atoms of elements with the same number of protons but a different number of neutrons are called isotopes.
Electrons are particles that spin around the nucleus. Traditionally, this is thought of as analogous to planets orbiting around the...
Table of contents
- Cover
- Title Page
- Copyright
- Preface
- Acknowledgments
- Acronyms
- Nomenclature
- About the companion website
- 1 Basic principles
- 2 Image weighting and contrast
- 3 Spin-echo pulse sequences
- 4 Gradient-echo pulse sequences
- 5 Spatial encoding
- 6 k-Space
- 7 Protocol optimization
- 8 Artifacts
- 9 Instrumentation
- 10 MRI safety
- Glossary
- Index
- End User License Agreement
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access MRI in Practice by Catherine Westbrook,John Talbot in PDF and/or ePUB format, as well as other popular books in Medicine & Medical Technology & Supplies. We have over one million books available in our catalogue for you to explore.