
- English
- ePUB (mobile friendly)
- Available on iOS & Android
About this book
This book focuses on the materials used for fuel cells, solar panels, and storage devices, such as rechargeable batteries.
Fuel cell devices, such as direct methanol fuel cells, direct ethanol fuel cells, direct urea fuel cells, as well as biological fuel cells and the electrolytes, membranes, and catalysts used there are detailed. Separate chapters are devoted to polymer electrode materials and membranes.
With regard to solar cells, the types of solar cells are detailed, such as inorganic-organic hybrid solar cells, solar powered biological fuel cells, heterojunction cells, multi-junction cells, and others. Also, the fabrication methods are described. Further, the electrolytes, membranes, and catalysts used there are detailed. The section that is dealing with rechargeable batteries explains the types of rechargeable devices, such as aluminum-based batteries, zinc batteries, magnesium batteries, and lithium batteries. Materials that are used for cathodes, anodes and electrolytes are detailed.
The text focuses on the basic issues and also the literature of the past decade. Beyond education, this book may serve the needs of polymer specialists as well as other specialists, e.g., materials scientists, electrochemical engineers, etc., who have only a passing knowledge of these issues, but need to know more.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
Chapter 1
Fuel Cells
- Alkaline fuel cells (AFC),
- Phosphoric acid fuel cells (PAFC),
- Polymer electrolyte fuel cells (PEFC),
- Molten carbonate fuel cell (MAFC), and
- Solid oxide fuel cells (SOFC).
1.1 Conventional Fuel Cells
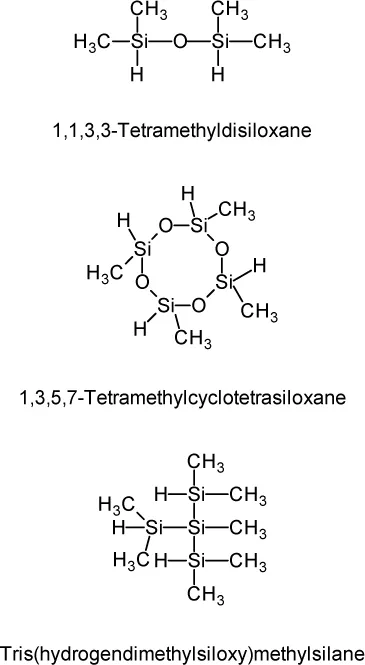
1.1.1 Sealing Material for Solid Polymer Fuel Cell Separator
| Compound |
| 1,1,3,3-Tetramethyldisiloxane 1,3,5,7-Tetramethylcyclotetrasiloxane Tris(hydrogendimethylsiloxy)methylsilane Tris(hydrogendimethylsiloxy)phenylsilane Methylhydrogencyclo poly(siloxane) Methylhydrogensiloxane-dimethylsiloxane cyclic copolymers Methylhydrogen poly(siloxane) capped at both ends with trimethylsiloxy groups Dimethylsiloxane-methylhydrogensiloxane copolymers capped at both ends with trimethylsiloxy groups Dimethyl poly(siloxane) capped at both ends with dimethylhydrogensiloxy groups Dimethylsiloxane-methylhydrogensiloxane copolymers capped at both ends with dimethylhydrogensiloxy groups Methylhydrogensiloxane-diphenylsiloxane copolymers capped at both ends with trimethylsiloxy groups Methylhydrogensiloxane-diphenylsiloxane-dimethylsiloxane copolymers capped at both ends with trimethylsiloxy groups Methylhydrogensiloxane-methylphenylsiloxane-dimethylsiloxane copolymers capped at both ends with trimethylsiloxy groups Methylhydrogensiloxane-dimethylsiloxane-diphenylsiloxane copolymers capped at both ends with dimethylhydrogensiloxy groups Methylhydrogensiloxane-dimethylsiloxane-methylphenylsiloxane copolymers capped at both ends with dimethylhydrogensiloxy groups Copolymers consisting of (CH3)2HsiO   |
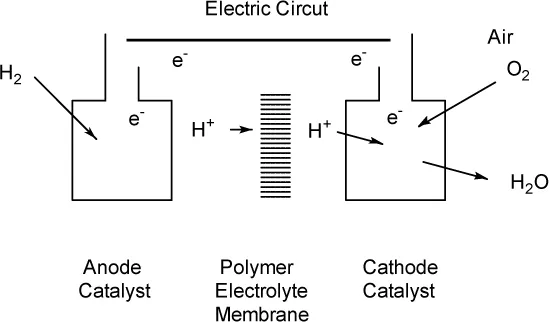
1.1.2 Water Management in a Polymer Electrolyte Fuel Cell
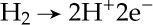
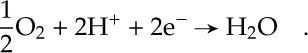
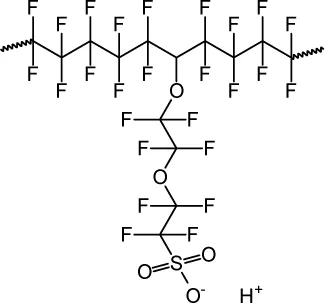
- Optical imaging (11–13),
- Magnetic resonance imaging (MRI) (14),
- Neutron radiography (15–18), and
- X-ray imaging techniques.
Table of contents
- Cover
- Title page
- Copyright page
- Preface
- Chapter 1: Fuel Cells
- Chapter 2: Polymer Electrodes
- Chapter 3: Polymer Membranes
- Chapter 4: Solar Cells
- Chapter 5: Rechargeable Batteries
- Index
- End User License Agreement