
eBook - ePub
Atmospheric Aerosols
Life Cycles and Effects on Air Quality and Climate
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Atmospheric Aerosols
Life Cycles and Effects on Air Quality and Climate
About this book
The book describes the morphological, physical and chemical properties of aerosols from various natural and anthropogenic sources to help the reader better understand the direct role of aerosol particles in scattering and absorbing short- and long-wave radiation.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Atmospheric Aerosols by Claudio Tomasi, Sandro Fuzzi, Alexander Kokhanovsky, Claudio Tomasi,Sandro Fuzzi,Alexander Kokhanovsky in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Atomic & Molecular Physics. We have over one million books available in our catalogue for you to explore.
Information
Chapter 1
Primary and Secondary Sources of Atmospheric Aerosol
Claudio Tomasi and Angelo Lupi
1.1 Introduction
Atmospheric aerosols are suspensions of any substance existing in the solid and/or liquid phase in the atmosphere (except pure water) under normal conditions and having a minimum stability in air assuring an atmospheric lifetime of at least 1 h. Generated by natural sources (i.e., wind-borne dust, sea spray, volcanic debris, biogenic aerosol) and/or anthropogenic activities (i.e., sulfates and nitrates from industrial emissions, wind-forced mineral dust mobilized in areas exploited for agricultural activities, fossil fuel combustion, and waste and biomass burning), aerosol particles range in size from a few nanometers to several tens of microns. As a result of internal cohesive forces and their negligible terminal fall speeds, aerosol particles can first assume sizes appreciably larger than the most common air molecules and subsequently increase to reach sizes ranging most frequently from less than 10−3 to no more than 100 µm (Heintzenberg, 1994). Particles with sizes smaller than 20–30 Å (1 Å = 10−10 m) are usually classified as clusters or small ions, while mineral and tropospheric volcanic dust particles with sizes greater than a few hundred microns are not considered to belong to the coarse aerosol class, since they have very short lifetimes. Aerosol particles grown by condensation to become cloud droplets are not classified as aerosols, although a cloud droplet needs a relatively small aerosol particle acting as a condensation nucleus for its formation under normal atmospheric conditions. Similarly, precipitation elements such as rain droplets, snowflakes, and ice crystals are not classified as aerosols (Heintzenberg, 1994). Although present in considerably lower concentrations than those of the main air molecules, aerosol particles play a very important role in numerous meteorological, physical, and chemical processes occurring in the atmosphere, such as the electrical conductivity of air, condensation of water vapor on small nuclei and subsequent formation of fog and cloud droplets, acid rains, scattering, and absorption of both incoming solar (shortwave) radiation and thermal terrestrial (longwave) radiation. The interaction processes between atmospheric aerosols and the downwelling and upwelling radiation fluxes of solar and terrestrial radiation at the surface play a major role in defining the radiation budget of our planet and, hence, the Earth's climate (Chylek and Coakley, 1974).
To give an idea of the shape of an aerosol particle suspended in dry air, a schematic representation of a particle originating from the aggregation of various kinds of particulate matter fragments is shown in Figure 1.1. It consists of several small unit structures of different chemical composition and origin (soluble acid substances, sodium chloride crystals of marine origin, ammonium sulfates, insoluble carbonaceous matter, insoluble mineral dust, and insoluble organic substances), held together by interparticle adhesive forces in such a way that an aerosol particle behaves as a single unit in suspension. Thus, the same particle often contains distinct homogeneous entities, which are internally mixed to form aggregates of different components.
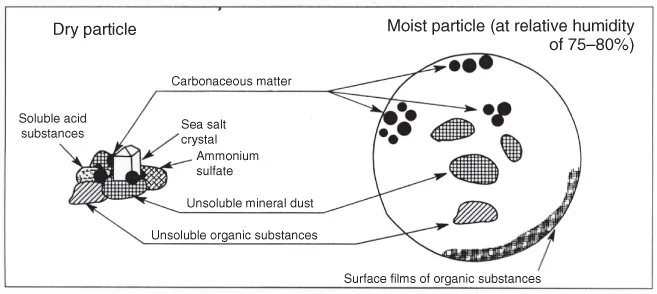
Figure 1.1 Schematic representation of an aerosol particle for dry air conditions (left) and humid air (for relative humidity (RH) = 75–80%) conditions (right), consisting of particulate matter pieces of soluble (i.e., soluble acid substances, sea-salt crystal, ammonium sulfates) and insoluble substances (carbonaceous matter, mineral dust, organic substances), which remain suspended inside the moist particle gradually growing by condensation until becoming a water droplet with soluble salts, acids, and organic compounds. (Adapted from a draft presented by Gottfried Hänel in a seminar given in 1985 at the FISBAT-CNR Institute, Bologna, Italy.)
The insoluble carbonaceous and organic substances often consist of gas-borne particulate matter pieces from incomplete combustion, which predominantly contain carbon and other combustion-produced materials. When the surrounding air relative humidity (RH) increases to reach values higher than 65–70%, the same particle (containing soluble substances) grows gradually by condensation of water vapor to become a water droplet in which pieces of insoluble matter are suspended, as can be seen in the (b) of Figure 1.1 (see also Hänel, 1976), while the various soluble materials reach different solution states as a result of their appreciably differing deliquescence properties. In this way, an internally mixed particle evolves assuming the characteristics of an aggregate consisting of different particulate phases. Figure 1.1 also shows that dry aerosol particles can often exhibit irregular shapes, which can considerably differ from the spherical one. Thus, the size of each real aerosol particle is generally evaluated in terms of an “equivalent” diameter a, for which the volume of such an ideal spherical particle is equal to that of the real particle.
Aerosol particles cover a size range of more than five orders of magnitude, with “equivalent” sizes ranging from 5 × 10−3 to 2.5 µm for fine particles and greater than 2.5 µm for coarse particles (Hinds, 1999). The fine particles include both (i) the so-called Aitken nuclei, having sizes mainly ranging from 5 × 10−3 to 5 × 10−2 µm, and (ii) the so-called “accumulation” particles having sizes ranging from 5 × 10−2 to about 2 µm. In this classification, it is worth mentioning that (i) the nuclei constitute the most important part of the so-called ultrafine particles (which have sizes <10−1 µm) and mainly form through condensation of hot vapors during combustion processes and/or nucleation of atmospheric gaseous species to form fresh particles and (ii) the accumulation particles are mainly generated through coagulation of small particles belonging to the nuclei class and condensation of vapors onto existing particles, inducing them to grow appreciably. Consequently, the particle concentration within this size subrange increases, and the accumulation mode becomes gradually more evident, so named because the particle removal mechanisms are poorly efficient in limiting the concentration of such an intermediate-size class of particles. Therefore, such particles have longer residence times than the nuclei, and ...
Table of contents
- Cover
- Wiley Series in Atmospheric Physics and Remote Sensing
- Title Page
- Copyright
- Table of Contents
- List of Contributors
- Preface
- Foreword
- Acknowledgments
- Chapter 1: Primary and Secondary Sources of Atmospheric Aerosol
- Chapter 2: Aerosol Nucleation in the Terrestrial Atmosphere
- Chapter 3: Coagulation, Condensation, Dry and Wet Deposition, and Cloud Droplet Formation in the Atmospheric Aerosol Life Cycle
- Chapter 4: Chemical Composition of Aerosols of Different Origin
- Chapter 5: Aerosol Optics
- Chapter 6: Aerosol Models
- Chapter 7: Remote Sensing of Atmospheric Aerosol
- Chapter 8: Aerosol and Climate Change: Direct and Indirect Aerosol Effects on Climate
- Chapter 9: Aerosol and Air Quality
- Chapter 10: Impact of the Airborne Particulate Matter onthe Human Health
- Chapter 11: Aerosol Impact on Cultural Heritage: Deterioration Processes and Strategies for Preventive Conservation
- Index
- End User License Agreement