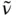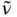Charge transfer is a fundamental step in many chemical and biological processes, including photosynthesis and metabolism [1–4]. The recent technological applications of charge-transfer–based materials include organic-light-emitting diodes (OLEDs), solar energy conversion, fluorescence sensing, nonlinear optical (NLO) materials, and so on [5–7]. The charge-transfer process may be divided into two broad categories. The transfer of charge from an electron-rich donor moiety to an electron-poor acceptor part located in different molecules is known as intermolecular charge-transfer process. However, if the donor and the acceptor belong to the same molecule, the phenomenon is called intramolecular charge-transfer (ICT) process. The ICT process generally occurs in the photoexcited state which a molecule reaches due to absorption of light of proper wavelength. The photoexcitation facilitates transfer of an electron from one part of a molecule/ion to its other part in the excited state, which makes the charge distribution in the excited state markedly different from that in the ground state. The through-bond ICT occurs in molecules in which the donor and the acceptor groups are connected through a π-electron bridge (
Figure 1.1). In some rare cases, an intramolecular through-space charge transfer is observed, where the transfer of charge through the conjugative path is denied but donor and acceptor groups are in a favorable position for charge transfer. Although the intermolecular interaction mediated by through-space charge transfer dictates the properties of many π-stacked molecular systems, studies of intramolecular through-space charge transfer is scarce. In π-conjugated organic molecules comprising electron donor (D) and acceptor (A) subunits, the process has attracted a lot of attention due to their immense technological implications in organic electronics and photovoltaics [5–14]. Materials based on such organic molecules are potential candidates for use in OLEDs, field-effect transistors, dye-sensitized solar cells, to name a few. In this book, our focus is mainly on the excited-state ICT in stable organic molecules as well as in inorganic complexes. A few examples of the electron-transfer process in biomolecules have also been discussed. The signatures of ICT, spectroscopic
techniques, and theoretical tools employed to study this process are also mentioned. It is now known that excited-state ICT in organic molecules may give rise to dual emission in its electronic spectrum. The peak seen at the blue end of the emission spectrum is generally believed to be arising from a locally excited (LE) state of the molecule, while the peak at the red end is generally assumed to bear the signature of an ICT species formed in the excited state. The ICT process generally occurs in polar solvents and the Stokes-shifted ICT fluorescence is observed due to solvent stabilization in the excited state [15]. In
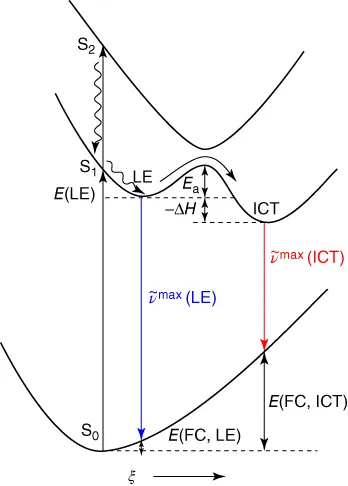
Figure 1.2, the potential energy surfaces (PES) for the ground state S
0 and the first two excited states (S
1, S
2) have been depicted along with the LE and ICT states. The vertical coordinate represents energy, while the horizontal coordinate (
ξ) comprises all molecular changes accompanying the LE → ICT reaction, such as changes in bond lengths and bond angles. In the given example, excitation of the molecule leads it to the S
2 state, which relaxes through internal
conversion to the equilibrated LE state. The ICT reaction proceeds from the LE to the ICT state that has a reaction barrier
Ea and an enthalpy difference of Δ
H. Fluorescence from the LE and ICT states reaches the corresponding Franck–Condon states
E(FC, LE) and
E(FC, ICT). This gives rise to dual fluorescence with emission maxima of
max(LE) and
max(ICT), respectively. Lippert and coworkers reported the dual fluorescence in 4-
N,
N-dimethylaminobenzonitrile (DMABN) for the first time in 1962 [16]. The debate continued regarding the origin of the dual emission of DMABN for some years. Most of the studies accepted that the observed dual emission from DMABN is due to excited-state ICT from the dimethylamine to the cyano group through the π-electron bridge. Later on, many congeners of DMABN were put under the scanner for deciphering or decoding the nature of the ICT process and its dynamics. Many experimental or theoretical studies or both have been devoted to investigate the charge-transfer mechanism in different organic molecules. The early experimental and theoretical results on the ICT process support a twisted intramolecular charge-transfer state (called TICT state) [16]. In the framework of the TICT model, the dual emission of an ICT probe originates from the primary excited, called LE, state as well as from the ICT state. The ICT state is accessible only by an adiabatic photoreaction from the LE state that includes rotational motion around the bond connecting the donor and acceptor moieties. If there is no energy barrier between the LE and ICT states, the excited-state relaxation can occur extremely rapidly, resulting in emission from the ICT species only. Although the TICT mechanism is till date the most popular concept in describing the structure of the excited state, this model was challenged by several groups. Later on, several other models, like planar intramolecular charge transfer (PICT), rehybridized intramolecular charge transfer (RICT), and wagging intramolecular charge transfer (WICT), were proposed by several groups to explain their experimental results. For example, Domcke and coworkers put forward a RICT model to account for the formation of the ICT state of DMABN and its analogs. Later on, the formation of a charge-transfer state from a rigid molecule,
N-phenylpyrrole (PP) and its free analog, fluorazene phenylpyrrole (FPP) put a question mark on the validity of the TICT model. The similarity in spectral signatures of these molecules could not be explained using the TICT model. This led to the proposal of a PICT mechanism to explain the spectroscopic response of the aforesaid molecules. There are several other examples that support the fact that the ICT state of the molecule does not need to be twisted. The origin and drawbacks of some of these models have been described in
Chapter 2.
Another point regarding the formation of the ICT state that is still under intense debate is the pathways of the charge-transfer process, that is, the mechanism through which the ICT process in a molecule occurs. Several high-level calculations and state-of-the-art experimental techniques have been used to settle this issue. In spite of several studies that describe the PES of the ICT process, this issue is yet to be settled amicabl...