
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Classical Methods in Structure Elucidation of Natural Products
About this book
The structures of many natural products are given in standard textbooks on organic chemistry as 'established facts'. Yet for those natural products whose structures were determined between 1860 and 1960 by classical chemical methods, the lines of evidence are frequently buried under any number of investigations that led to dead ends and to revised structure assignments. Since very little is known about the structure clarification of these products at present, this volume serves to shed light once again on the achievements of previous generations of chemists, who worked with minimal experimental tools.
The selection of the 25 representative examples is subjective and arbitrary, dictated by the author's pleasure in recovering fundamental milestones in organic chemistry, with each chapter devoted to one organic compound. The time period covered, however, is more precisely defined: 1860 represents the advent of structure theory, prior to which there was no conceptual framework to address the 'structure' of a compound. One hundred years later, 1960 approximately marks the change from classical structure elucidation to the era in which structure elucidation is mainly based on spectroscopic evidence and X-ray crystallography. Since the emphasis of this work is on classical structure elucidation, work performed later than 1960 is only considered in exceptional cases.
Rather than simply provide a history of structure elucidation of particular natural products, the author combines results from historic experiments to trace a line of evidence for those structures that are nowadays accepted as established. This line of evidence may follow the path put forward by the original contributors, yet in some cases the experimental facts have been combined to form another, hopefully shorter, line of evidence. As a result, readers are able to ascertain for themselves the 'facts behind the established structure assignments' of a number of important natural products.
The selection of the 25 representative examples is subjective and arbitrary, dictated by the author's pleasure in recovering fundamental milestones in organic chemistry, with each chapter devoted to one organic compound. The time period covered, however, is more precisely defined: 1860 represents the advent of structure theory, prior to which there was no conceptual framework to address the 'structure' of a compound. One hundred years later, 1960 approximately marks the change from classical structure elucidation to the era in which structure elucidation is mainly based on spectroscopic evidence and X-ray crystallography. Since the emphasis of this work is on classical structure elucidation, work performed later than 1960 is only considered in exceptional cases.
Rather than simply provide a history of structure elucidation of particular natural products, the author combines results from historic experiments to trace a line of evidence for those structures that are nowadays accepted as established. This line of evidence may follow the path put forward by the original contributors, yet in some cases the experimental facts have been combined to form another, hopefully shorter, line of evidence. As a result, readers are able to ascertain for themselves the 'facts behind the established structure assignments' of a number of important natural products.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Classical Methods in Structure Elucidation of Natural Products by Reinhard W. Hoffmann in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Organic Chemistry. We have over one million books available in our catalogue for you to explore.
Information
Part I
Compounds with only Oxygen Functionalities
Chapter 1
Ascorbic Acid
Figure 1.1 Albert Szent-Györgyi (b. 16.9.1893–d. 22.10.1986), in 1911, started to study medicine at the University of Budapest. This was followed by his cosmopolitan years in Prague, Berlin, Hamburg, Leiden, Groningen, Cambridge, and Rochester, before accepting a chair at the University of Szeged in 1930. In 1947, he moved to the Marine Biology Laboratories in Woods Hole (MA) in the United States. In 1937, Albert Szent-Györgyi was awarded the Nobel Prize for Medicine for his research on vitamin C.Source: J.W. McGuire, https://commons.wikimedia.org/wiki/File:Albert_Szent-Gy%C3%B6rgyi_cropped.webp. CC-public domain.

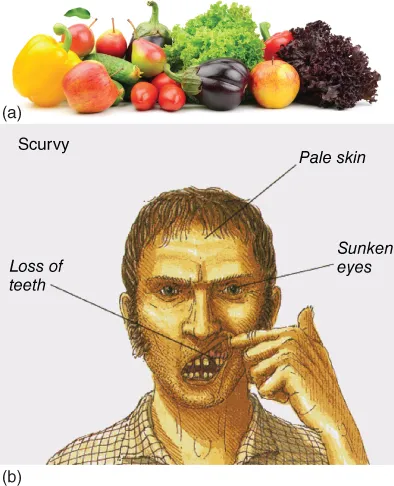
A strongly reducing substance, C6H8O6, was isolated from the adrenal glands in 1928 by Szent-Györgyi [1]. This substance was later identified as vitamin C, the essential food constituent, the lack of which leads to scurvy (in French, “scorbut”). Hence, this substance was given the name ascorbic acid [2].
Figure 1.2 (a) Fruits containing vitamin C. (b) Signs of scurvy. Source: (a) Serg64/Shutterstock, www.gettyimages.com (b) Dorling Kindersley Ltd, Gütersloh, Germany. With kind permission of Dorling Kindersley Ltd, Gütersloh, Germany.
The highly oxygenated nature of this substance indicated a relationship with carbohydrates, and, indeed, ascorbic acid showed a positive Molisch test. The molecular formula suggests ascorbic acid to be a dehydrogenated (−4H) hexose.
Information Box 1
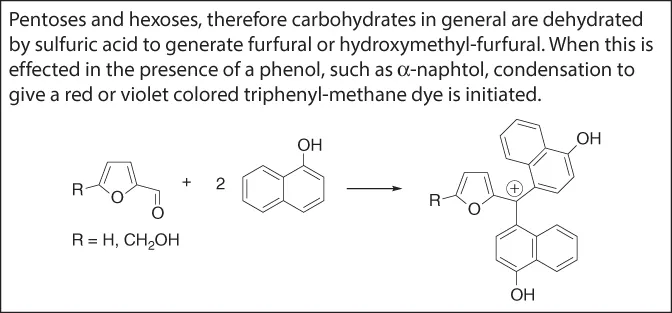
Molisch Test for Pentoses and Hexoses [3, 4].
Preliminary Findings.
Upon treatment with chloro(triphenyl)methane, ascorbic acid readily formed a trityl ether [5]. Hence, ascorbic acid contains a primary alcohol function. Upon treatment with HCl, ascorbic acid formed furfural quantitatively [6]. Accordingly, ascorbic acid contains at least five C-atoms in a linear chain.
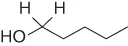
Ascorbic acid readily formed an acetonide [7]. It should, therefore, be a 1,2- or 1,3-diol. Finally, it was established with a Zerewitinoff test that ascorbic acid contains four H-atoms active toward Grignard reagent CH3MgI [8].
Information Box 2
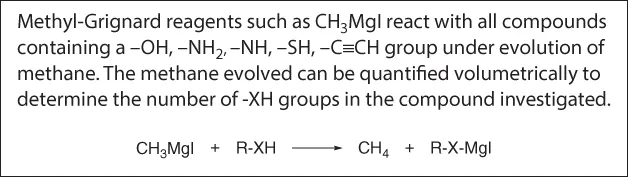
Zerewitinoff Test for Active Hydrogen [9].
As the name implies, ascorbic acid is acidic, with a pKA value of 4.1 [5]. Ascorbic acid thus readily yielded a sodium salt C6H7O6Na [8]. Upon reaction of ascorbic acid with CH2N2, two (acidic) OH groups were methylated to give a dimethoxy compound 1.1 C8H12O6 [8, 10].
Ascorbic acid as well as its sodium salt gave a strong positive result for the Fe3+ color test for enols [8], whereas that of the dimethoxy compound was negative. Therefore, at least one of the acidic H-atoms in ascorbic acid belongs to an enol, and the other one may belong to a second enol or to a carboxylic acid.
Cleavage of ascorbic acid into smaller fragments was accomplished by oxidation: upon oxidation with NaOI, 1 equiv. of oxalic acid was obtained [6]. Oxidation of ascorbic acid by KMnO4 furnished oxalic acid and a 2,3,4-trihydroxybutanoic acid 1.2; Scheme 1.1 [6, 11].
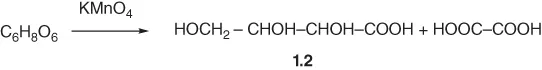
Scheme 1.1
Since there are two diastereomeric forms of 2,3,4-trihydroxybutanoic acid, the relative configuration of 1.2 was addressed at this point. To this end, compound 1.2 was permethylated with (MeO)2SO2/KOH, and the methyl ester obtained was converted with NH3 to a crystalline amide (Scheme 1.2).
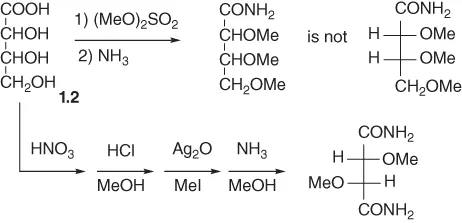
Scheme 1.2
This amide turned out to be different from the known amide of erythro-2,3,4-trimethoxy-butanoic acid. Hence, ...
Table of contents
- Cover
- Title Page
- Copyright
- Table of Contents
- Preface
- Hundred Years of Structure Elucidation
- Part I: Compounds with only Oxygen Functionalities
- Part II: Compounds with Nitrogen and Oxygen Functionalities
- Part III: Compounds with Additional Functionalities
- Part IV: Compounds without Heteroatom-Functionalities
- Part V: Can You Do it Yourself?
- Index
- End User License Agreement