
Mass Spectrometry
An Applied Approach
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Mass Spectrometry
An Applied Approach
About this book
Provides a comprehensive description of mass spectrometry basics, applications, and perspectives
Mass spectrometry is a modern analytical technique, allowing for fast and ultrasensitive detection and identification of chemical species. It can serve for analysis of narcotics, counterfeit medicines, components of explosives, but also in clinical chemistry, forensic research and anti-doping analysis, for identification of clinically relevant molecules as biomarkers of various diseases. This book describes everything readers need to know about mass spectrometry—from the instrumentation to the theory and applications. It looks at all aspects of mass spectrometry, including inorganic, organic, forensic, and biological MS (paying special attention to various methodologies and data interpretation). It also contains a list of key terms for easier and faster understanding of the material by newcomers to the subject and test questions to assist lecturers.
Knowing how crucial it is for young researchers to fully understand both the power of mass spectrometry and the importance of other complementary methodologies, Mass Spectrometry: An Applied Approach teaches that it should be used in conjunction with other techniques such as NMR, pharmacological tests, structural identification, molecular biology, in order to reveal the true function(s) of the identified molecule.
- Provides a description of mass spectrometry basics, applications and perspectives of the technique
- Oriented to a broad audience with limited or basic knowledge in mass spectrometry instrumentation, theory, and its applications in order to enhance their competence in this field
- Covers all aspects of mass spectrometry, including inorganic, organic, forensic, and biological MS with special attention to application of various methodologies and data interpretation
- Includes a list of key terms, and test questions, for easier and faster understanding of the material
Mass Spectrometry: An Applied Approach is highly recommended for advanced students, young scientists, and anyone involved in a field that utilizes the technique.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
1
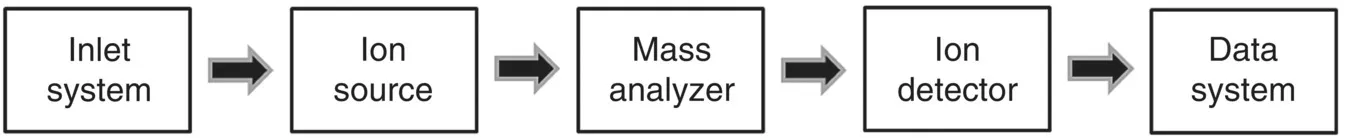
Introduction
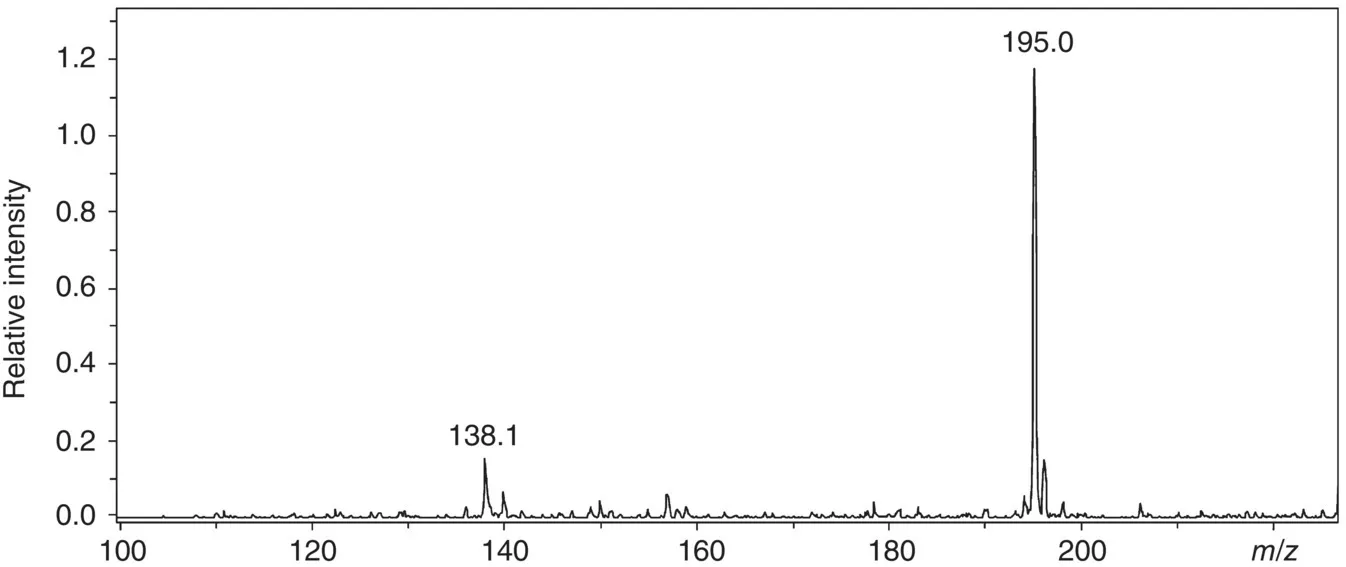
- Speed of analysis.
- High sensitivity, reaching femto‐/attomolar level.
- Simultaneous analysis of many components of mixtures.
- Ability to obtain information on the structure of the compounds (including amino acid sequence) and posttranslational modifications.
- Possibility of combining with separation techniques (e.g. gas and liquid chromatography, capillary electrophoresis, isotachophoresis).
- Quantitative analysis.
- Analysis of the elemental composition.
- Analysis of isotopic composition.
- Unambiguous identification of the substance.
- It provides the relationship between the retention time of the substance and the intensity of the peaks on the spectrum (quantitative analysis).
- It also provides information on substances eluted from the column at the same time.
- It moreover provides detailed information about the structure of the compounds (identification of unknown components).
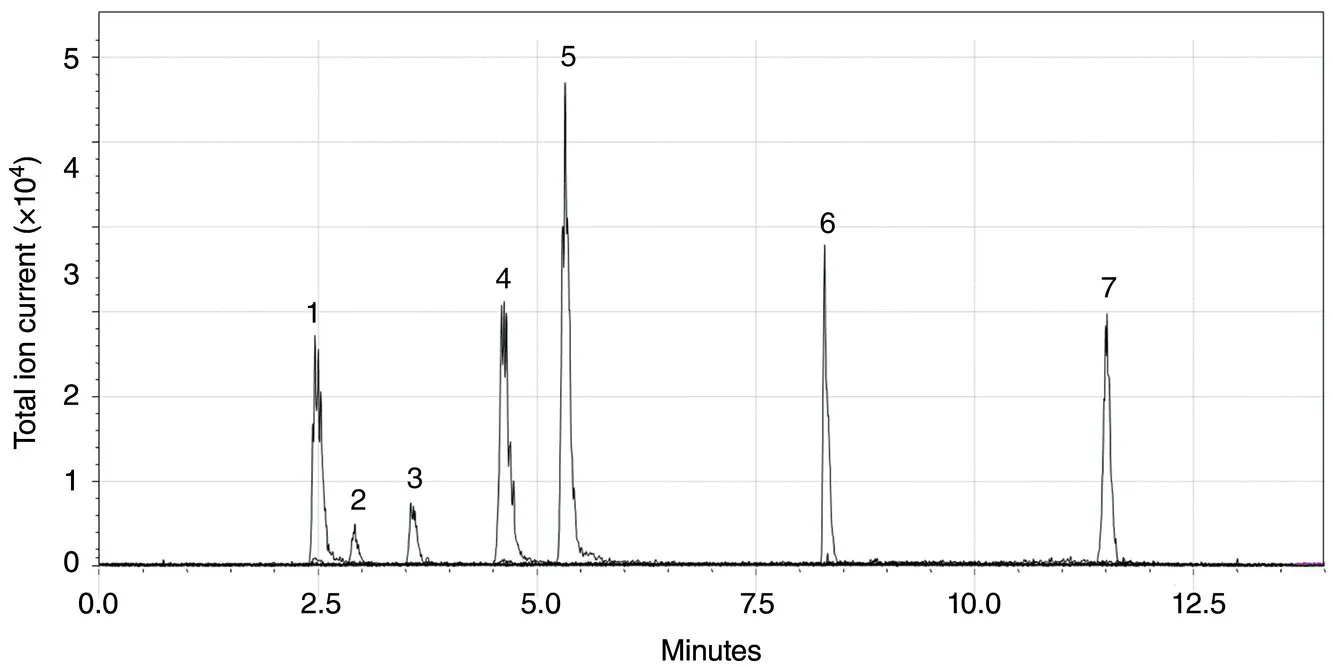
Table of contents
- Cover
- Table of Contents
- List of Contributors
- Preface
- 1 Introduction
- 2 A Brief History of Mass Spectrometry
- 3 Basic Definitions
- 4 Instrumentation
- 5 Hyphenated Techniques
- 6 Mass Spectrometry Imaging
- 7 Tandem Mass Spectrometry
- 8 Mass Spectrometry Applications
- 9 Appendix
- 10 Abbreviations
- Index
- End User License Agreement