
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Delivery Systems for Tuberculosis Prevention and Treatment
About this book
Provides a review of novel pharmaceutical approaches for Tuberculosis drugs
- Presents a novel perspective on tuberculosis prevention and treatment
- Considers the nature of disease, immunological responses, vaccine and drug delivery, disposition and response
- Multidisciplinary appeal, with contributions from microbiology, immunology, molecular biology, pharmaceutics, pharmacokinetics, chemical and mechanical engineering
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Delivery Systems for Tuberculosis Prevention and Treatment by Anthony J. Hickey in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Industrial & Technical Chemistry. We have over one million books available in our catalogue for you to explore.
Information
1
Introduction: A Guide to Treatment and Prevention of Tuberculosis Based on Principles of Dosage Form Design and Delivery
A.J. Hickey
RTI International, RTP, NC, USA
1.1 Background
Tuberculosis has been a scourge of mankind for millennia. The discovery by Koch of the causative organism Mycobacterium tuberculosis at the end of the nineteenth century was hailed as the discovery that would rapidly lead to its eradication [1]. Despite the speed of development of a vaccine, attenuated Mycobacterium bovis (bacille Calmette Guerin), and the discovery of a therapeutic drug within only a few decades, circumstances that could not have been foreseen with respect to new strains, multiple-drug resistance and co-infection with human immunodeficiency virus, have rendered the disease a more complicated challenge than originally envisaged.
As the twentieth century progressed physicians were horrified to discover that the vaccine was not universally protective and that resistance to the drug of choice, streptomycin, was increasing rapidly [2]. These observations led to further activities in both the realm of vaccine and drug development, the latter being the more clinically successful but the former yielding much need information on the pathogen, the host immunity and pathogenesis of disease.
During this period pharmacy and pharmaceutical dosage form design were also entering a golden age. Manufacturing of drug products or compounding, which was traditionally an activity that took place in a pharmacy, was transferred to an industrial setting. Commercial products involving a variety of dosage form were being standardized to allow production on a scale previously unknown. The introduction of legislation regulating the quality of products, particularly to address adulteration and ensure safety, commenced most notably in the 1930s with the Food Drug and Cosmetics Act of the United States [3]. In the latter half of the twentieth century the underlying physical chemistry and chemical engineering required to manufacture under rigorously controlled conditions that ensured the quality, uniformity, efficacy and safety of the product were developed.
With this background it is noteworthy that the parallel developments in dosage form and tuberculosis (TB) treatment led to their convergence in the early part of the twentieth century when reproducible drug delivery could only be achieved by oral administration (tablets and capsules) or parenteral administration (injection). As a consequence, other routes and means of delivery were rarely, if ever, considered for the delivery of drugs or vaccines. This can be contrasted with the products of biotechnology developed in the late twentieth century for which both oral and parenteral administration were rarely feasible. Of course, the ease of delivery and the required dose were the leading reasons for the selection of these routes of administration.
There was a brief period in the middle of the twentieth century when the absence of new drugs and the increase in drug resistance led to studies of inhaled therapy for tuberculosis but the development of new drugs resulted in this approach being abandoned and only revisited during times when there were no apparent oral and parenteral dosage forms to meet the immediate challenge. Figure 1.1 presents the number of publications that can be found in the accessible literature for the period since the initial rise in drug-resistant tuberculosis in the 1940s. A subsequent peak appears following the rise in human immunodeficiency virus co-infected patients and multiple-drug-resistant tuberculosis requiring alternative therapeutic strategies.
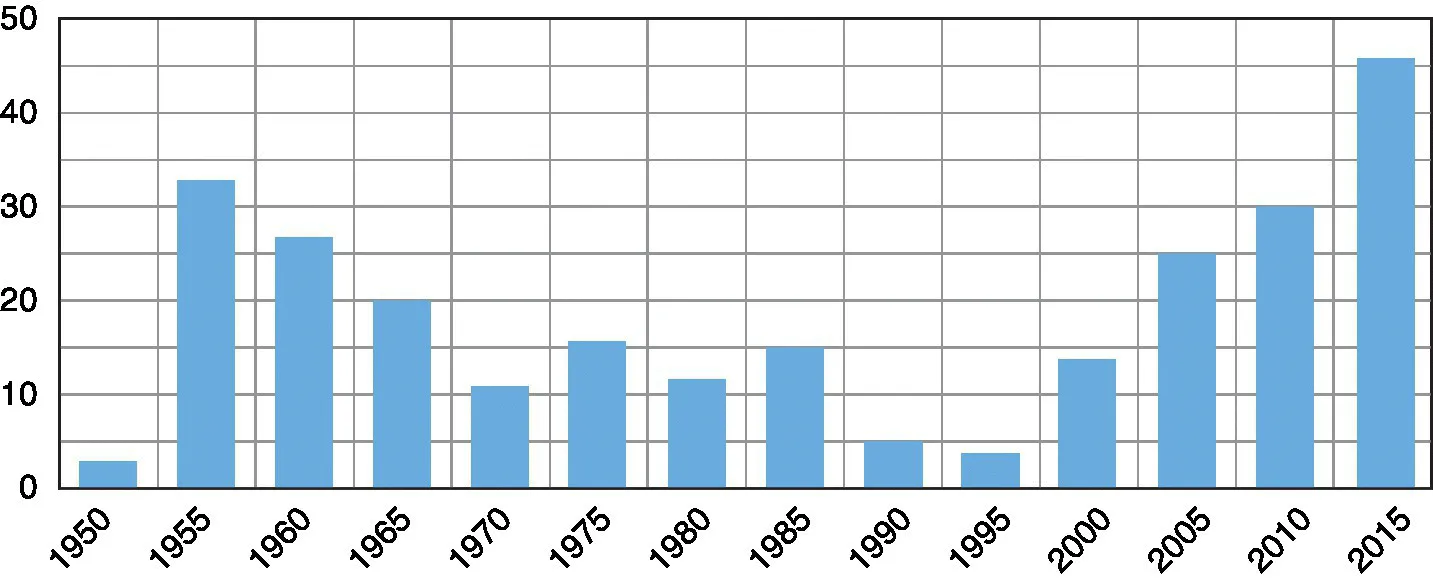
Figure 1.1 Reports of Aerosol Delivery Extracted from PubMed from the earliest citations in the modern literature
1.2 Dosage Form Classification
The route of administration by which drugs are delivered dictates the dosage form employed. The United States Pharmacopeia has classified therapeutic products in terms of three tiers: route of administration, dosage forms and performance test which captures all conventional and most novel strategies for disease treatment as shown in Figure 1.2 [4]. The performance measure of significance for the majority of dosage forms is the dissolution rate which, together with the biological parameter of permeability for those drugs presented at mucosal sites, dictates the appearance of the drug in the systemic circulation and ultimately its therapeutic effect.
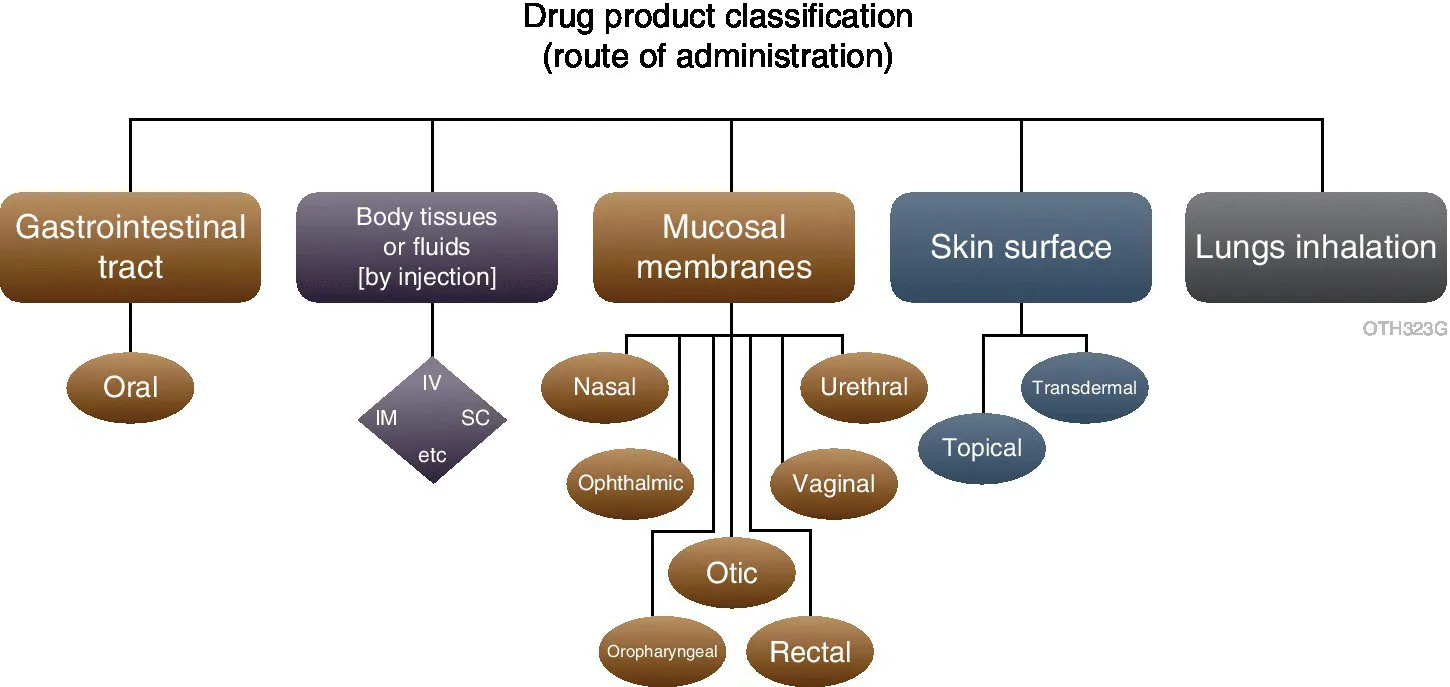
Figure 1.2 United States Pharmacopeia Taxonomy of Dosage Forms structured from: Tier 1 – Route of Administration; through Tier 2 – Dosage Form to; Tier 3 – Performance (not shown).
(Modified from ref. [4] Courtesy of Margareth Marques and the USP)
1.2.1 Dosage Forms
It would not be possible to do justice to the science and technology underpinning the wide range of dosage forms available for drug delivery. However, to put those used in the treatment and prevention of tuberculosis in context a brief review of the key components and processes involved may be helpful to the reader.
1.2.1.1 Solid Oral Dosage Forms
These consist of a mixture of powders each of which is intended to confer a desirable property on the dosage form that leads to effective manufacture, drug delivery and therapeutic effect [5, 6].
In addition to the drug substance which must be well characterized, glidants help the powder flow which aids in filling, surfactants enhance dissolution and diluents are considered inert bulking agents that assist in metering small quantities of drug during filling and may help in compaction. Binding agents, as the name suggests, help in binding all components into a granule or tablet to preserve the integrity of the dosage form on storage and prior to administration. The common dosage forms are capsules and tablets that differ in that the former consists of a powder or granulated loose fill while the latter requires compaction [5, 6]. The most common capsule is prepared with gelatin and filled with the optimized formulation of drug in excipients to allow for stability on storage and reproducible and efficacious dose delivery. Tablets also contain the drug and excipient compacted into a single solid dosage form that has desired performance properties in terms of stability, dissolution, dose delivery and efficacy. Biopharmaceutical considerations are of great significance to the disposition of drugs from solid oral dosage forms. Their behavior under the wide range of pH conditions (1–8) in the gastro-intestinal tract and an understanding of the influence of anatomy and physiology on local residence time and regions of absorption are significant considerations in optimization of the dosage form. Relatively recently the publication of Lipinski’s rules [7] and the biopharmaceutical classification system [8] have been an enormous help in the selection of drugs and requirements of formulations that correlate with successful drug delivery by the oral route of administration.
1.2.1.2 Parenteral Dosage Forms
These are intended for injection either directly into the blood circulation [intravenous (IV)] or at a site from which the drug can readily be transported to the vasculature as would occur following subcutaneous or intramuscular administration [9]. There are other infrequently employed (intraperitoneal) or specialized (intrathecal or intratumoral) sites of injection that are not relevant to tuberculosis therapy. The key elements of a parenteral dosage form are the requirement for a formulation suitable for delivery from a syringe through a needle to the intended site. The formulation can range from simple solutions to a variety of dispersed systems (emuls...
Table of contents
- Cover
- Title Page
- Table of Contents
- List of Contributors
- Foreword
- Advances in Pharmaceutical Technology: Series Preface
- Preface
- 1 Introduction
- Section 1: Pathogen and Host
- Section 2: Immunological Intervention
- Section 3: Drug Treatment
- Section 4: Alternative Approaches
- Section 5: Future Opportunities
- Section 6: Clinical Perspective
- Index
- End User License Agreement