
eBook - ePub
Instructor's Guide and Solutions Manual to Organic Structures from 2D NMR Spectra, Instructor's Guide and Solutions Manual
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Instructor's Guide and Solutions Manual to Organic Structures from 2D NMR Spectra, Instructor's Guide and Solutions Manual
About this book
The text Organic Structures from 2D NMR Spectra contains a graded set of structural problems employing 2D-NMR spectroscopy. The Instructors Guide and Solutions Manual to Organic Structures from 2D NMR Spectra is a set of step-by-step worked solutions to every problem in Organic Structures from 2D NMR Spectra. While it is absolutely clear that there are many ways to get to the correct solution of any of the problems, the instructors guide contains at least one complete pathway to every one of the questions. In addition, the instructors guide carefully rationalises every peak in every spectrum in relation to the correct structure. The Instructors Guide and Solutions Manual to Organic Structures from 2D NMR Spectra: Is a complete set of worked solutions to the problems contained in Organic Structures from 2D NMR Spectra. Provides a step-by-step description of the process to derive structures from spectra as well as annotated 2D spectra indicating the origin of every cross peak. Highlights common artefacts and re-enforces the important characteristics of the most common techniques 2D NMR techniques including COSY, NOESY, HMBC, TOCSY, CH-Correlation and multiplicity-edited C-H Correlation. This guide is an essential aid to those teachers, lecturers and instructors who use Organic Structures from 2D NMR as a text to teach students of Chemistry, Pharmacy, Biochemistry and those taking courses in Organic Chemistry.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Instructor's Guide and Solutions Manual to Organic Structures from 2D NMR Spectra, Instructor's Guide and Solutions Manual by L. D. Field,A. M. Magill,H. L. Li in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Spectroscopy & Spectrum Analysis. We have over one million books available in our catalogue for you to explore.
Information
Problem 1
Question:
The 1H and 13C{1H} NMR spectra of 1-iodopropane (C3H7I) recorded in CDCl3 solution at 298 K and 400 MHz are given below.
The 1H NMR spectrum has signals at δ 0.99 (H3), 1.84 (H2) and 3.18 (H1) ppm.
The 13C{1H} NMR spectrum has signals at δ 9.6 (C1), 15.3 (C3) and 26.9 (C2) ppm.
Also given on the following pages are the 1H–1H COSY, 1H–13C me-HSQC, 1H–13C HMBC and INADEQUATE spectra. For each 2D spectrum, indicate which correlation gives rise to each cross-peak by placing an appropriate label in the box provided (e.g. H1 → H2, H1 → C1).
Solution:

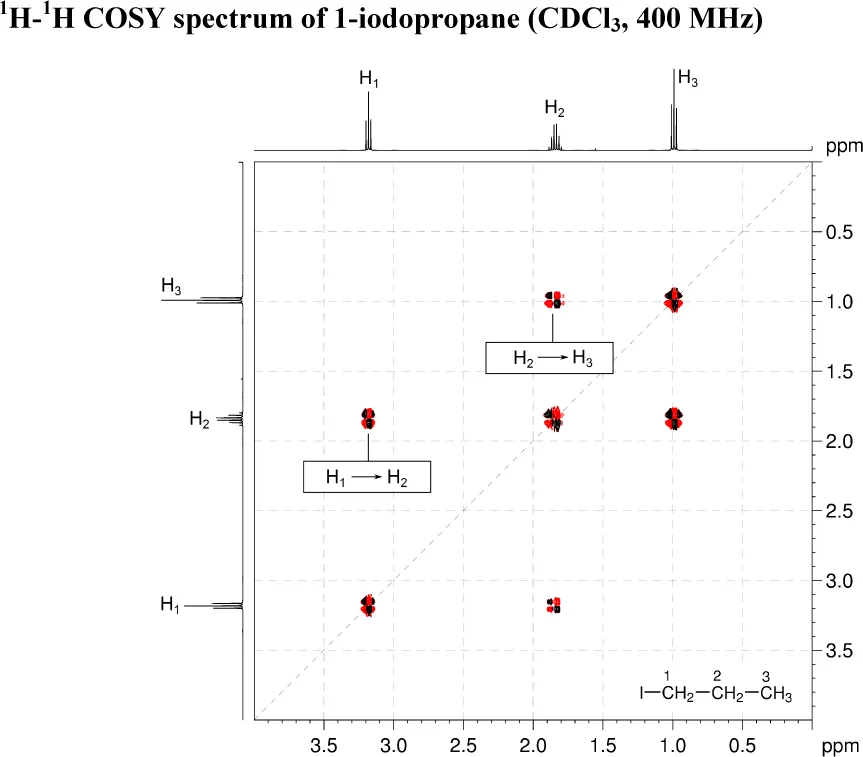
1. 1H–1H COSY spectra show which pairs of protons are coupled to each other. The COSY spectrum is always symmetrical about a diagonal. In the COSY spectrum, there are two 3JH–H correlations above the diagonal (H1 → H2 and H2 → H3). There are no long-range correlations.
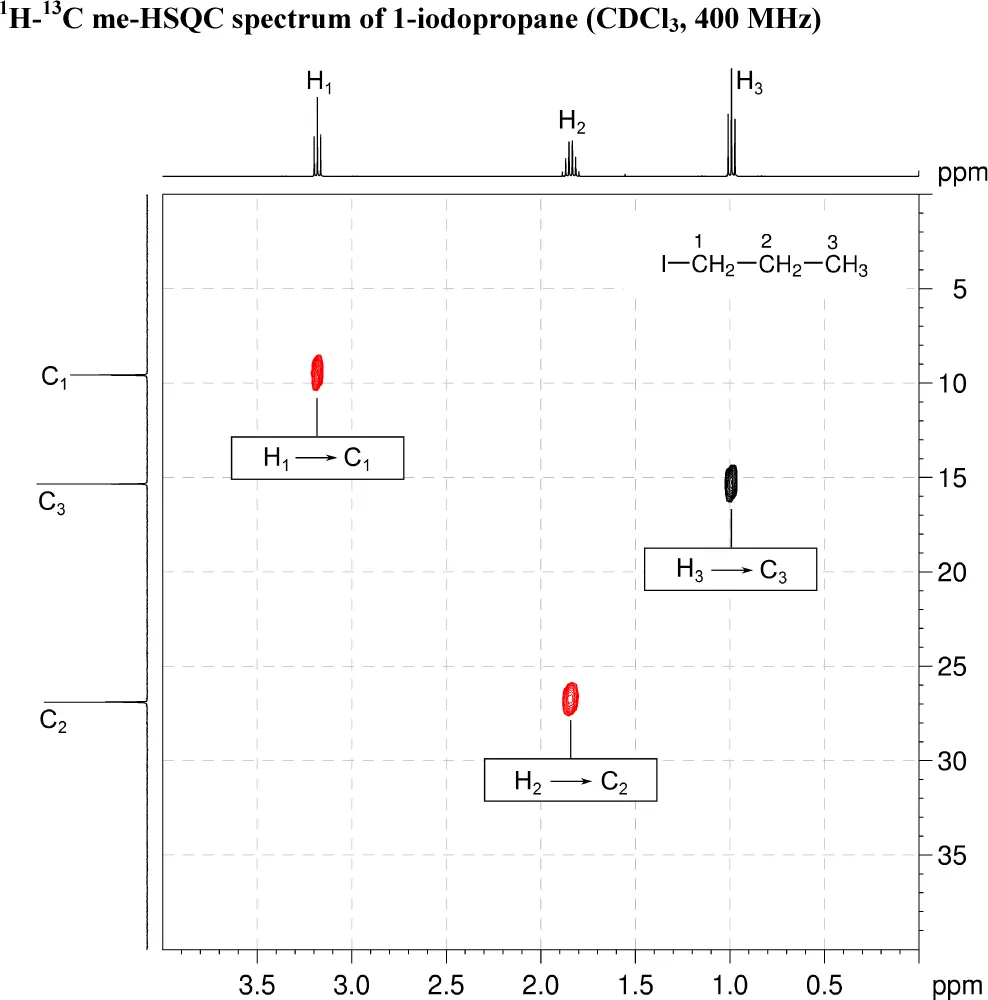
2. The 1H–13C me-HSQC spectrum shows direct (one-bond) correlations between proton and carbon nuclei, so there will be cross-peaks between H1 and C1, H2 and C2 and also between H3 and C3. As the spectrum is multiplicity edited, the cross-peaks corresponding to CH2 groups are shown in red and are of opposite phase to those for CH3 groups.
3. In HMBC spectra, remember that, for alkyl systems, both two- and three-bond C–H coupling can give rise to strong cross-peaks.
4. H1 correlates to C2 and C3. H2 correlates to C1 and C3. H3 correlates to C1 and C2.

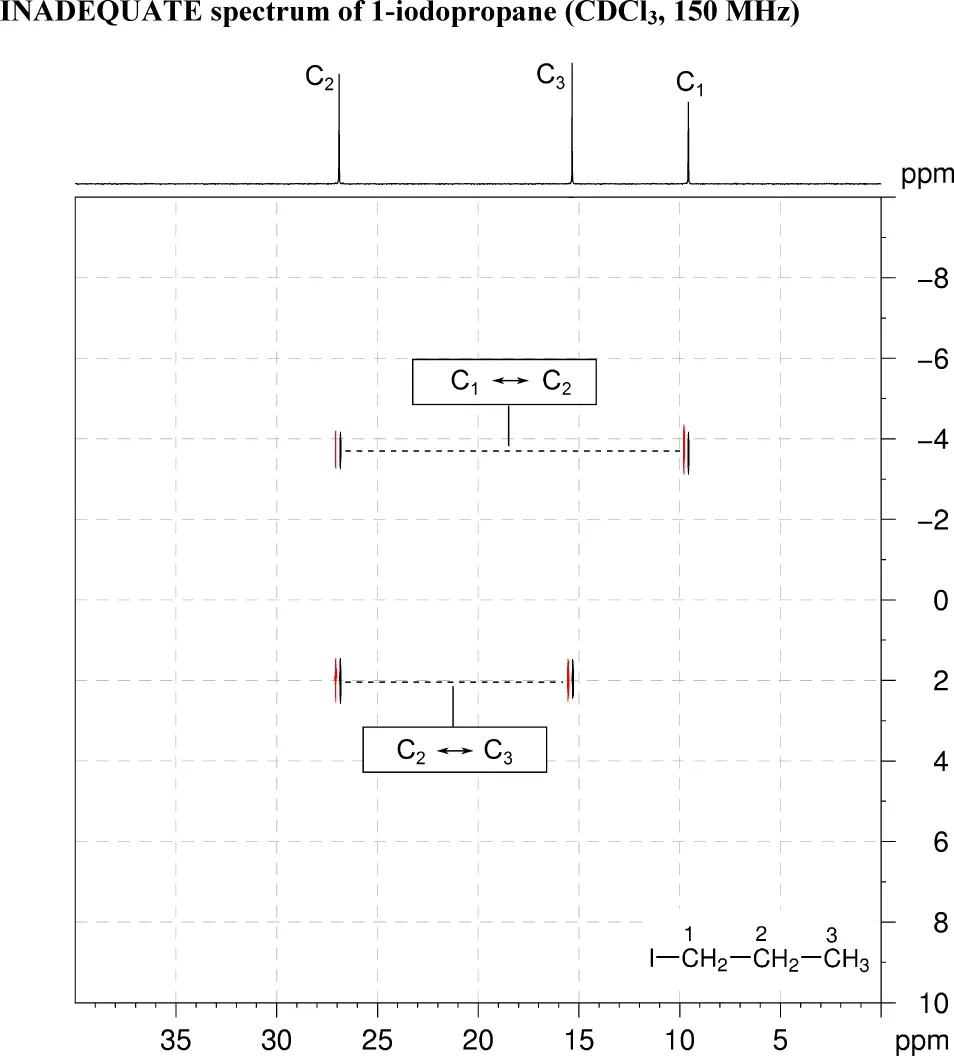
5. The INADEQUATE spectrum shows one-bond 13C–13C connectivity. There are correlations between C1 and C2, and C2 and C3.
Problem 2
Question:
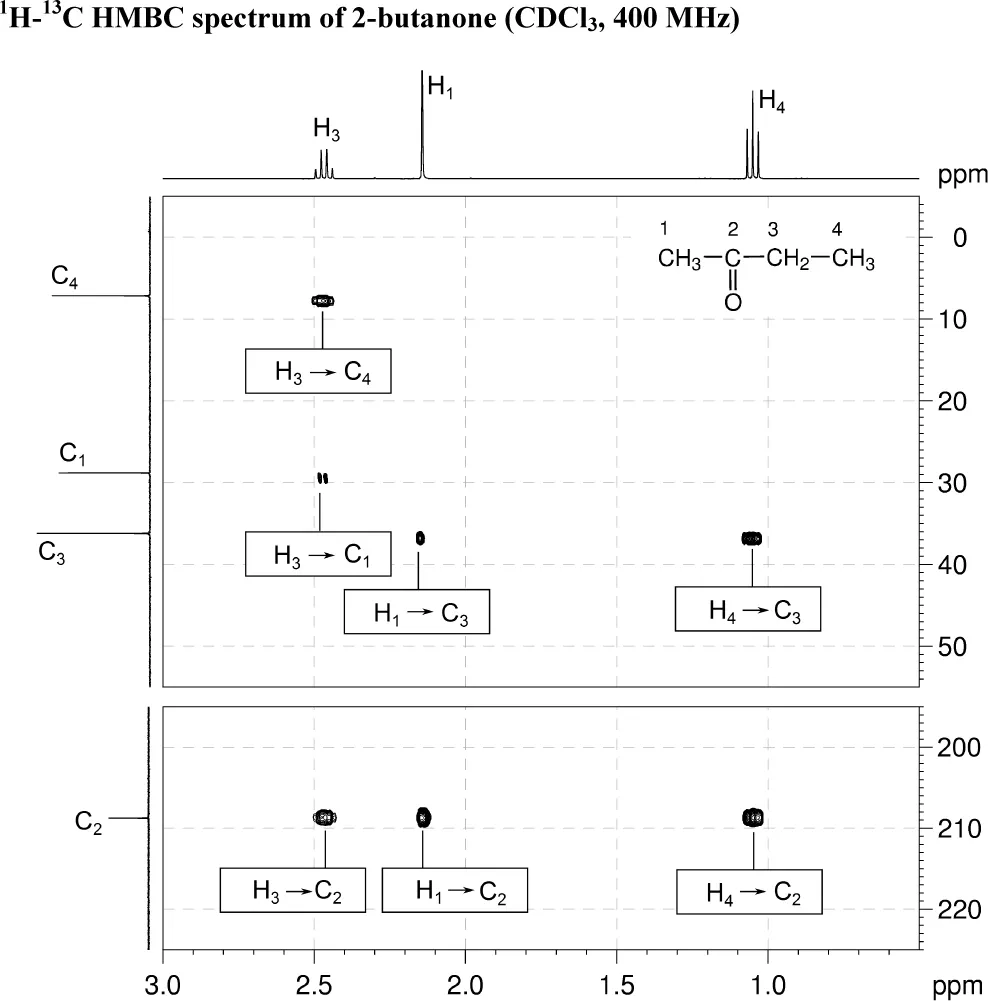
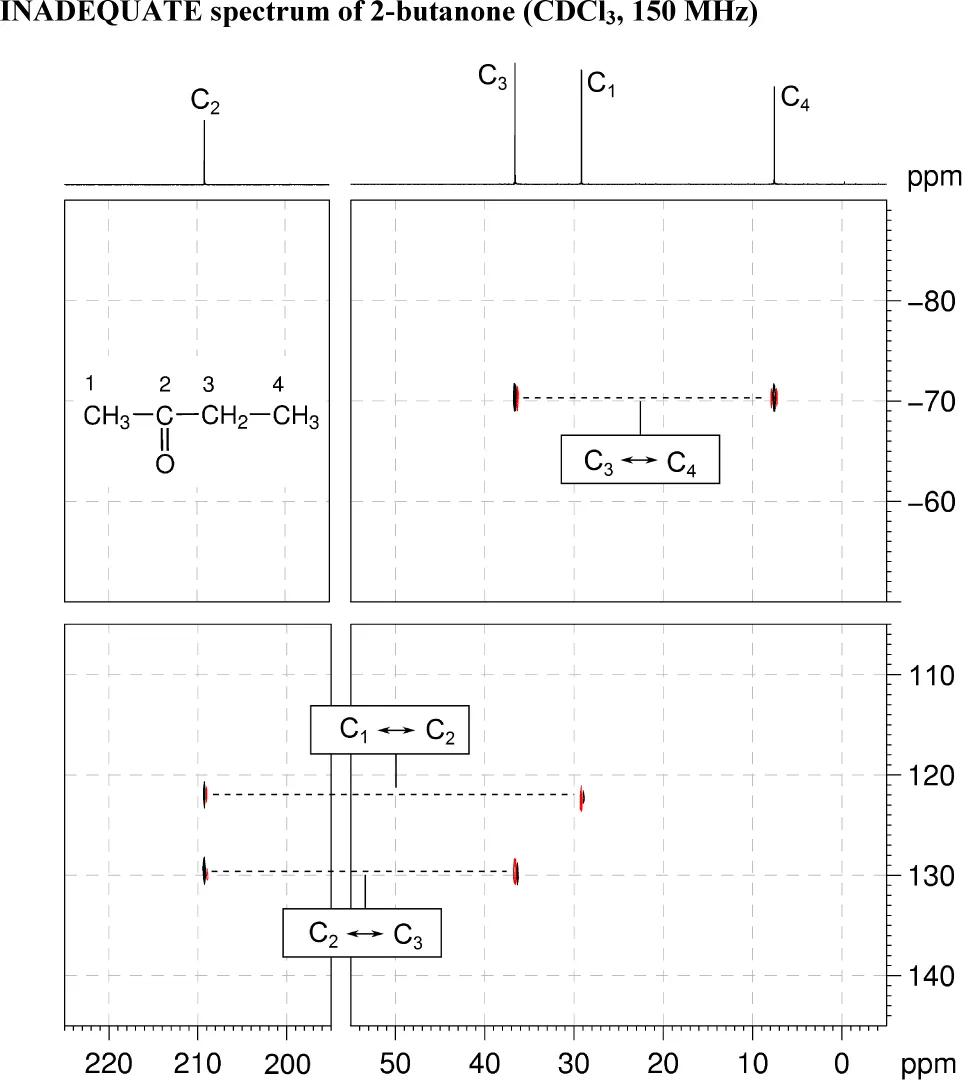
The 1H and 13C{1H} NMR spectra of 2-butanone (C4H8O) recorded in CDCl3 solution at 298 K and 400 MHz are given below.
The 1H NMR spectrum has signals at δ 1.05 (H4), 2.14 (H1) and 2.47 (H3) ppm.
The 13C{1H} NMR spectrum has signals at δ 7.2 (C4), 28.8 (C1), 36.2 (C3) and 208.8 (C2) ppm.
Also given on the following pages are the 1H–1H COSY, 1H–13C me-HSQC, 1H–13C HMBC and INADEQUATE spectra. For each 2D spectrum, indicate which correlation gives rise to each cross-peak by placing an appropriate label in the box provided (e.g. H1 → H2, H1 → C1).
Solution:

1. 1H–1H COSY spectra show which pairs of protons are coupled to each other. The COSY spectrum is always symmetrical about a diagonal. In the COSY spectrum, there is only one 3JH–H correlation above the diagonal (H3 → H4). There are no long-range correlations.

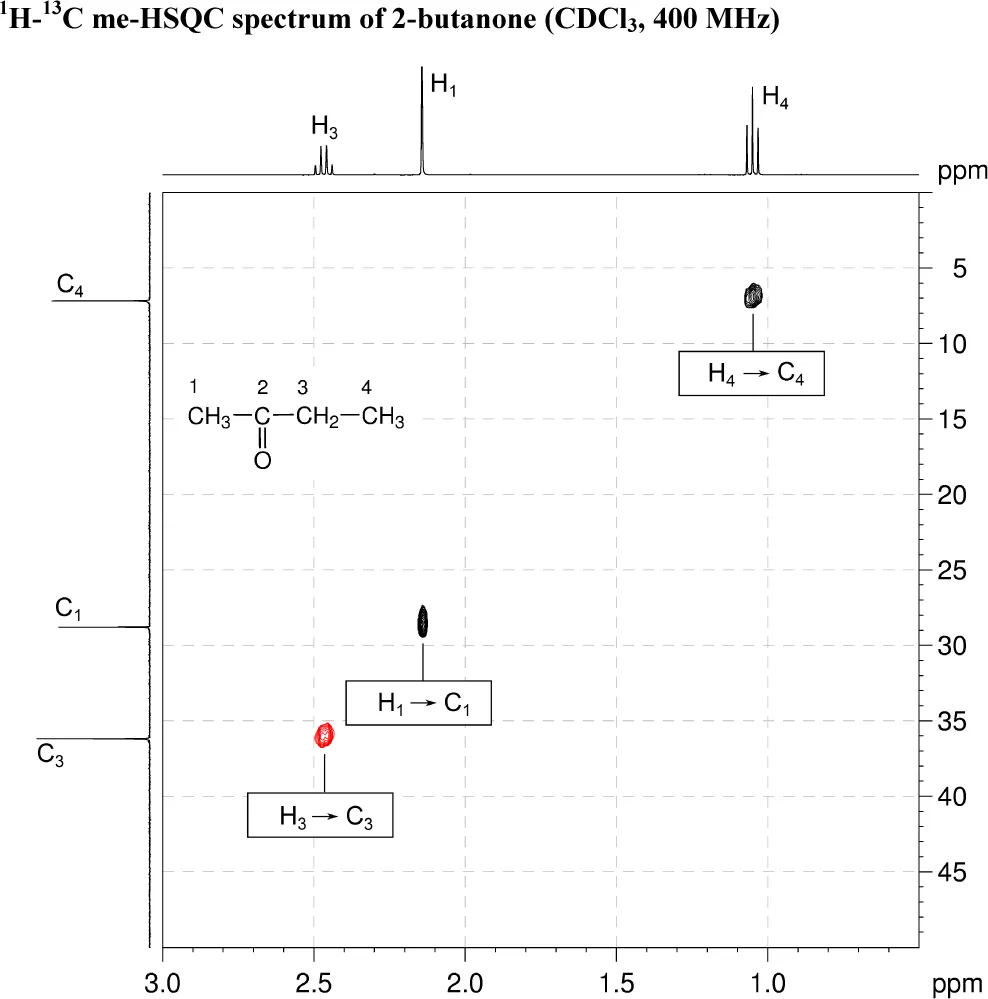
2. The 1H–13C me-HSQC spectrum shows direct (one-bond) correlations between proton and carbon nuclei, so there will be cross-peaks between H1 and C1, H3 and also between C3 and H4 and C4. As the spectrum is multiplicity edited, the cross-peaks corresponding to CH2 groups are shown in red and are of opposite phase to those for CH3 groups.
3. In HMBC spectra, remember that, for alkyl systems, both two- and three-bond coupling can give rise to strong cross-peaks. There are no one-bond C–H correlations.
4. H1 correlates to C2 and C3. H3 correlates to C1, C2 and C4. H4 correlates to C2 and C3.
5. The INADEQUATE spectrum shows one-bond 13C–13C connectivity. There are correlations between C1 and C2, C2 and C3 and C3 and C4.
Problem 3
Q...
Table of contents
- Cover
- Half Title page
- Title page
- Copyright page
- Preface
- Problem 1
- Problem 2
- Problem 3
- Problem 4
- Problem 5
- Problem 6
- Problem 7
- Problem 8
- Problem 9
- Problem 10
- Problem 11
- Problem 12
- Problem 13
- Problem 14
- Problem 15
- Problem 16
- Problem 17
- Problem 18
- Problem 19
- Problem 20
- Problem 21
- Problem 22
- Problem 23
- Problem 24
- Problem 25
- Problem 26
- Problem 27
- Problem 28
- Problem 29
- Problem 30
- Problem 31
- Problem 32
- Problem 33
- Problem 34
- Problem 35
- Problem 36
- Problem 37
- Problem 38
- Problem 39
- Problem 40
- Problem 41
- Problem 42
- Problem 43
- Problem 44
- Problem 45
- Problem 46
- Problem 47
- Problem 48
- Problem 49
- Problem 50
- Problem 51
- Problem 52
- Problem 53
- Problem 54
- Problem 55
- Problem 56
- Problem 57
- Problem 58
- Problem 59
- Problem 60
- Problem 61
- Problem 62
- Problem 63
- Problem 64
- Problem 65
- Problem 66