
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Organic Structures from 2D NMR Spectra
About this book
The derivation of structural information from spectroscopic data is now an integral part of organic chemistry courses at all Universities. Over recent years, a number of powerful two-dimensional NMR techniques (e.g. HSQC, HMBC, TOCSY, COSY and NOESY) have been developed and these have vastly expanded the amount of structural information that can be obtained by NMR spectroscopy. Improvements in NMR instrumentation now mean that 2D NMR spectra are routinely (and sometimes automatically) acquired during the identification and characterisation of organic compounds.
Organic Structures from 2D NMR Spectra is a carefully chosen set of more than 60 structural problems employing 2D-NMR spectroscopy. The problems are graded to develop and consolidate a student's understanding of 2D NMR spectroscopy. There are many easy problems at the beginning of the collection, to build confidence and demonstrate the basic principles from which structural information can be extracted using 2D NMR. The accompanying text is very descriptive and focussed on explaining the underlying theory at the most appropriate level to sufficiently tackle the problems.
Organic Structures from 2D NMR Spectra
- Is a graded series of about 60 problems in 2D NMR spectroscopy that assumes a basic knowledge of organic chemistry and a basic knowledge of one-dimensional NMR spectroscopy
- Incorporates the basic theory behind 2D NMR and those common 2D NMR experiments that have proved most useful in solving structural problems in organic chemistry
- Focuses on the most common 2D NMR techniques – including COSY, NOESY, HMBC, TOCSY, CH-Correlation and multiplicity-edited C-H Correlation.
- Incorporates several examples containing the heteronuclei 31P, 15N and 19F
Organic Structures from 2D NMR Spectra is a logical follow-on from the highly successful "Organic Structures from Spectra" which is now in its fifth edition. The book will be invaluable for students of Chemistry, Pharmacy, Biochemistry and those taking courses in Organic Chemistry.
Also available: http://www.wiley.com/WileyCDA/WileyTitle/productCd-111902725X. Instructors Guide and Solutions Manual to Organic Structures from 2D NMR Spectra
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
Chapter 1
NMR Spectroscopy Basics
1.1 THE PHYSICS OF NUCLEAR SPINS
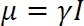
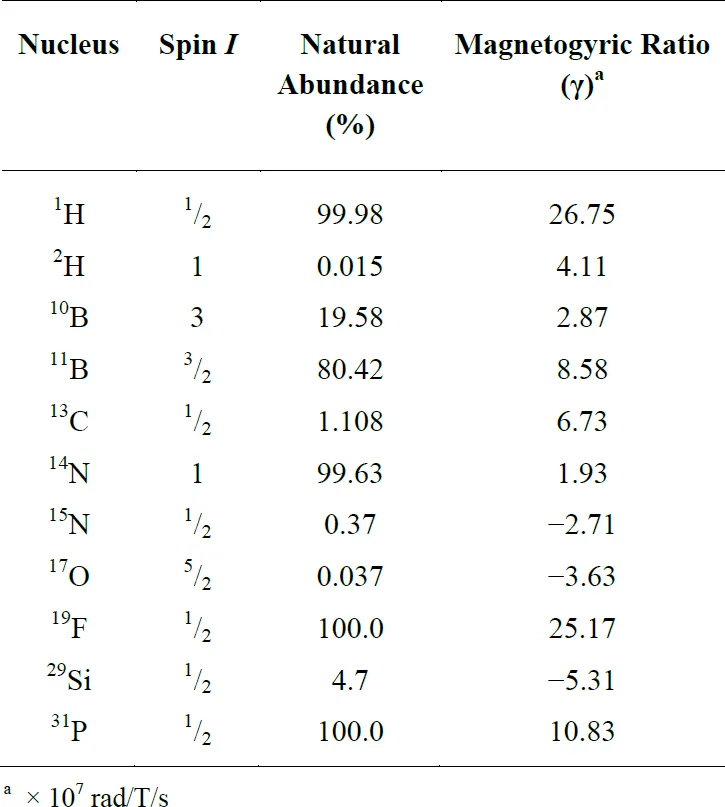
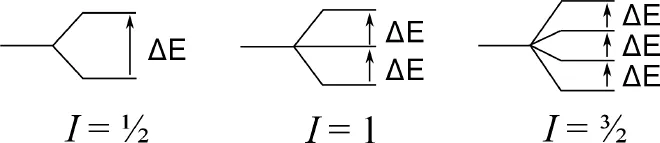
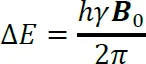
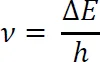
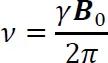

1.2 BASIC NMR INSTRUMENTATION AND THE NMR EXPERIMENT
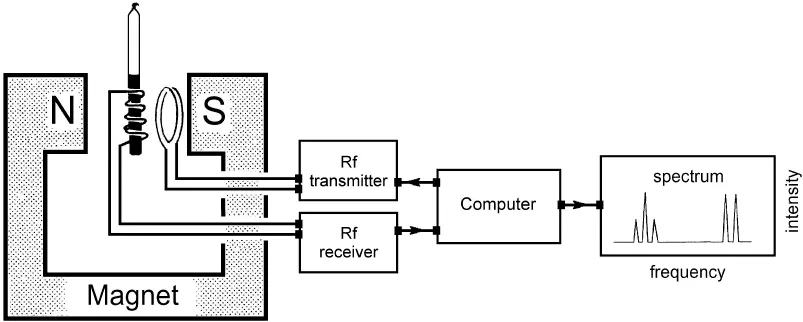
Chapter 2
One-Dimensional Pulsed Fourier Transform NMR Spectroscopy
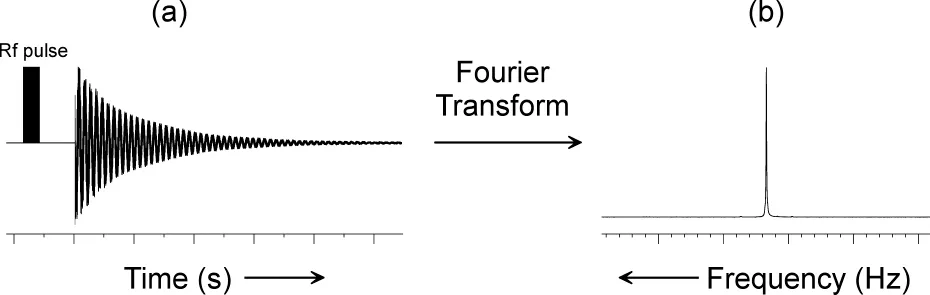
Table of contents
- Cover
- Half Title page
- Title page
- Copyright page
- Preface
- List of Figures
- List of Tables
- Chapter 1: NMR Spectroscopy Basics
- Chapter 2: One-Dimensional Pulsed Fourier Transform NMR Spectroscopy
- Chapter 3: Two-Dimensional NMR Spectroscopy
- Chapter 4: Miscellaneous Topics
- Chapter 5: Worked Examples
- Chapter 6: Problems
- Index