
eBook - ePub
Fundamentals of Ionic Liquids
From Chemistry to Applications
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Fundamentals of Ionic Liquids
From Chemistry to Applications
About this book
Written by experts who have been part of this field since its beginnings in both research and academia, this textbook introduces readers to this evolving topic and the broad range of applications that are being explored.
The book begins by examining what it is that defines ionic liquids and what sets them apart from other materials. Chapters describe the various types of ionic liquids and the different techniques used to synthesize them, as well as their properties and some of the methods used in their measurement. Further chapters delve into synthetic and electrochemical applications and their broad use as "Green" solvents. Final chapters examine important applications in a wide variety of contexts, including such devices as solar cells and batteries, electrochemistry, and biotechnology.
The result is a must-have resource for any researcher beginning to work in this growing field, including senior undergraduates and postgraduates.
The book begins by examining what it is that defines ionic liquids and what sets them apart from other materials. Chapters describe the various types of ionic liquids and the different techniques used to synthesize them, as well as their properties and some of the methods used in their measurement. Further chapters delve into synthetic and electrochemical applications and their broad use as "Green" solvents. Final chapters examine important applications in a wide variety of contexts, including such devices as solar cells and batteries, electrochemistry, and biotechnology.
The result is a must-have resource for any researcher beginning to work in this growing field, including senior undergraduates and postgraduates.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Fundamentals of Ionic Liquids by Douglas R. MacFarlane,Mega Kar,Jennifer M. Pringle in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Physical & Theoretical Chemistry. We have over one million books available in our catalogue for you to explore.
Information
Chapter 1
An Introduction to Ionic Liquids
1.1 Prologue
The benefits of using salts in their liquid form as electrolytes or reaction media have long been recognized. For example, Faraday developed his laws of electrolysis in the 1830s using molten metal halide salts. However, most researchers would rather avoid using solvents that require heating to hundreds of degrees. Therefore, while ‘high-temperature molten salts’ are extremely useful for certain applications, they are not in widespread use in research laboratories or industry. In contrast, ‘ionic liquids’ – and we will discuss the definition of these below – are proving to be very exciting for a very wide range of applications at more moderate temperatures. Particularly over the last decade, scientists working in many different areas of research have started to realize the special properties of ionic liquids (ILs) and to embrace the promise of these new materials. As more applications are discovered, and more families of ILs are developed, so the field continues to grow. Thousands of papers on ILs are now published every year, and, more importantly, increasing numbers of researchers are experimenting with ILs and discovering firsthand how their unique properties can help their work.
So here we are, just over 100 years since the first ‘room-temperature ionic liquid’ was discovered, at a point where these materials are now proving so promising and widely applicable that it is important for a broad range of scientists and engineers to have an appreciation of the basics of ILs. Thus, the purpose of this book is to serve as an introduction to the key concepts and applications of ILs for those venturing into this field for the first time. Hopefully, the book will also inspire further curiosity and enthusiasm for exploring these exciting and very unique materials.
Our goal in this book is to provide a thorough introduction to the field appropriate to the level of a finishing undergraduate science student or a beginning postgraduate student. Our emphasis is on illustrative examples and the background chemistry sufficient to understand the fundamentals of ILs and their applications. For further reading, we have referenced more extensive reviews where they exist. To provide background on fundamental concepts and methods that may not be readily accessible in standard textbooks, we have included Concept Toolbox items as breakout text boxes in various places throughout the chapters. This first chapter provides a broad overview of the field, the materials involved, their properties, and their applications, of which more details can be found later in this book.
1.2 The Definition of an Ionic Liquid
The phrase ‘ionic liquid’ was coined only relatively recently to refer to ambient-temperature liquid salts, and the definition has since been the subject of much discussion and some evolution. The most useful practical definition of an IL is
‘A liquid comprised entirely of ions.’
We can delve into this a little deeper. By this definition, is an IL different from a molten salt? The answer is: ‘No’ – the term ‘molten salt’ refers to the liquid phase of a crystalline salt, for example, NaCl. ‘Ionic Liquid’ covers that, but also covers a broader range of possibilities. Imagine a mixture of the two salts Na[fsi] and [C3mpyr][NTf2] (see Table 1.1 for an explanation of abbreviations). This mixture of salts is a liquid at room temperature and is properly called an IL by our definition. In fact an IL could contain a very large number of different ions. Note that, in common usage, the term ‘molten salt’ has also come to mean mixtures of salts, although the term itself clearly indicates a single compound.
Table 1.1 Glossary of structures and nomenclature abbreviations used in this book
| Quaternary cations | Abbreviation | Structure |
| Alkylmethylimidazolium | [Cnmim]+ |  |
| Alkyldimethylimidazolium | [Cndmim]+ |  |
| Alkylmethylpyrrolidinium | [Cnmpyr]+ |  |
| Alkylmethylpiperidinium | [Cnmpip]+ | 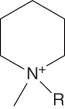 |
| Ammonium | [Nn,n,n,n]+ | 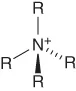 |
| Phosphonium | [Pn,n,n,n]+ | 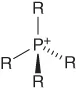 |
| Ether-functionalized | [NR,R,R,2O1]+, etc. | 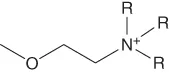 |
| Cholinium | [Ch]+ | 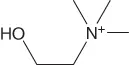 |
| Sulfonium | [SR,R′,R″]+ | 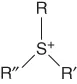 |
| Guanidinium | [Gdm]+ | 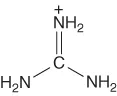 |
| Tetrafluoroborate | [BF4]− | 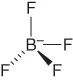 |
| Hexafluorophosphate | [PF6]− | 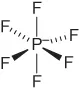 |
| Trifluoroacetate | [tfa]− | 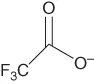 |
| Triflate | [OTf]− or [CF3SO3]− | 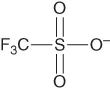 |
| Bis(fluorosulfonyl)imidea | [fsi]− or [N(SO2F)2]− | 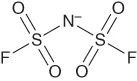 |
| Bis(trifluoromethanesulfonyl)imidea | [NTf2]− or [N(SO2CF3)2]− |  |
| Dicyanamide | [N(CN)2]− or [dca]− | 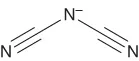 |
| Tetracyanoborate | [B(CN)4]− | 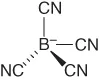 |
| Fluoroalkylphosphates | [fap]−, [efap]−, etc. | 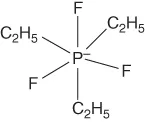 |
| Dihydrogenphosphate | [H2PO4]− or [dhp]− | 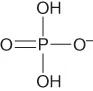 |
| Hydrogen sulfate | [HSO4]− | 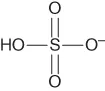 |
| p-Toluenesulfonate or tosylate | [Tos]− | 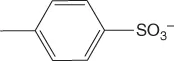 |
| Tetrahydroborate or borohydride | [BH4]− |  |
a Also referred to as ‘amides’.
In principle (in fact an important thermodynamic principle), the IL obtained by mixing Na[fsi] and [C3mpyr][NTf2] is exactly the same as that obtained by mixing the appropriate quantities of Na[NTf2] and [C3mpyr][fsi]. The points of origin are irrelevant in defining the IL; only the quantities of the individual ions present are important. In fact, such ILs with very high concentrations of Na or Li salts are proving to be highly effective as electrolytes for Na and Li batteries [1].
Some definitions of IL add a temperature range, such as ‘below 100 °C’, to the definition but this is not necessary. In fact, it is limiting to do so, since it can blinker our perspective on which compounds or mixtures may be useful for certain applications. Indeed, there are many, quite valuable, applications of ILs at temperatures above 100 °C, for example, the preparation of MnOx water oxidation catalysts by electrodeposition at 130 °C [2]. The key requirement for this is that the IL be a liquid at 130 °C. It is convenient if it is also liquid at room temperature, but it is not necessary for this to be the case and one should certainly not exclude from consideration compounds having melting points >100 °C for an application such as this. Similarly, a definition that includes ‘a salt having a melting point below 100 °C’ (or some other temperature such as room temperature) is also an unnecessary restriction because in some cases the melting point may be practically difficult to find and measure. The supercooling of liquids below their equilibrium melting points is a well-known phenomenon, and in some cases the liquid becomes so viscous that the crystalline phase never forms on a practical timescale. This is particularly true of mixtures of salts, which we have agreed are perfectly good ILs, because the melting points of individual compounds is often sharply depressed in mixtures. We will discuss ...
Table of contents
- Cover
- Title Page
- Copyright
- Table of Contents
- Chapter 1: An Introduction to Ionic Liquids
- Chapter 2: The Structure of Ions that Form Ionic Liquids
- Chapter 3: Structuring of Ionic Liquids
- Chapter 4: Synthesis of Ionic Liquids
- Chapter 5: Physical and Thermal Properties
- Chapter 6: Solvent Properties of Ionic Liquids: Applications in Synthesis and Separations
- Chapter 7: Electrochemistry of and in Ionic Liquids
- Chapter 8: Electrochemical Device Applications
- Chapter 9: Biocompatibility and Biotechnology Applications of Ionic Liquids
- Index
- End User License Agreement