
Handbook of Graphene, Volume 1
Growth, Synthesis, and Functionalization
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Handbook of Graphene, Volume 1
Growth, Synthesis, and Functionalization
About this book
This unique multidisciplinary 8-volume set focuses on the emerging issues concerning graphene materials and provides a shared platform for both researcher and industry.
The Handbook of Graphene comprises a set of 8 individual volumes that brings an interdisciplinary perspective to accomplish a more detailed understanding of the interplay between the synthesis, structure, characterization, processing, applications and performance of the advanced materials. The Handbook of Graphene comprises 140 chapters from world renowned experts.
Volume 1 is solely focused on Growth, Synthesis, and Functionalization of Graphene. Some of the important topics include but not limited to: Graphite in metallic materials-growths, structures and defects of spheroidal graphite in ductile iron; synthesis and quality optimization; methods of synthesis and physico-chemical properties of fluorographenes; graphene-SiC reinforced hybrid composite foam: response to high strain rate deformation; atomic structure and electronic properties of few-layer graphene on SiC(001); features and prospects for epitaxial graphene on SiC; graphitic carbon/graphene on Si(111) via direct deposition of solid-state carbon atoms: growth mechanism and film characterization; chemical reactivity and variation in electronical properties of graphene on Ni(111) and reduced graphene oxide; chlorophyll and graphene: a new paradigm of biomimetic symphony; graphene structures: from preparations to applications; three-dimensional graphene-based structures: production methods, properties and applications; electrochemistry of graphene materials; hydrogen functionalized graphene nanostructure material for spintronic application; the impact of uniaxial strain and defect pattern on magnetoelectronic and transport properties of graphene; exploiting graphene as an efficient catalytic template for organic transformations: synthesis, characterization and activity evaluation of graphene-based catalysts; exfoliated graphene based 2D materials; synthesis and catalytic behaviors; functionalization of graphene with molecules and/or nanoparticles for advanced applications; carbon allotropes "between diamond and graphite": how to create semiconductor properties in graphene and related structures.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
Chapter 1
Graphite in Metallic Materials Growths, Structures, and Defects of Spheroidal Graphite in Ductile Iron
Abstract
1.1 Graphite in Cast Irons
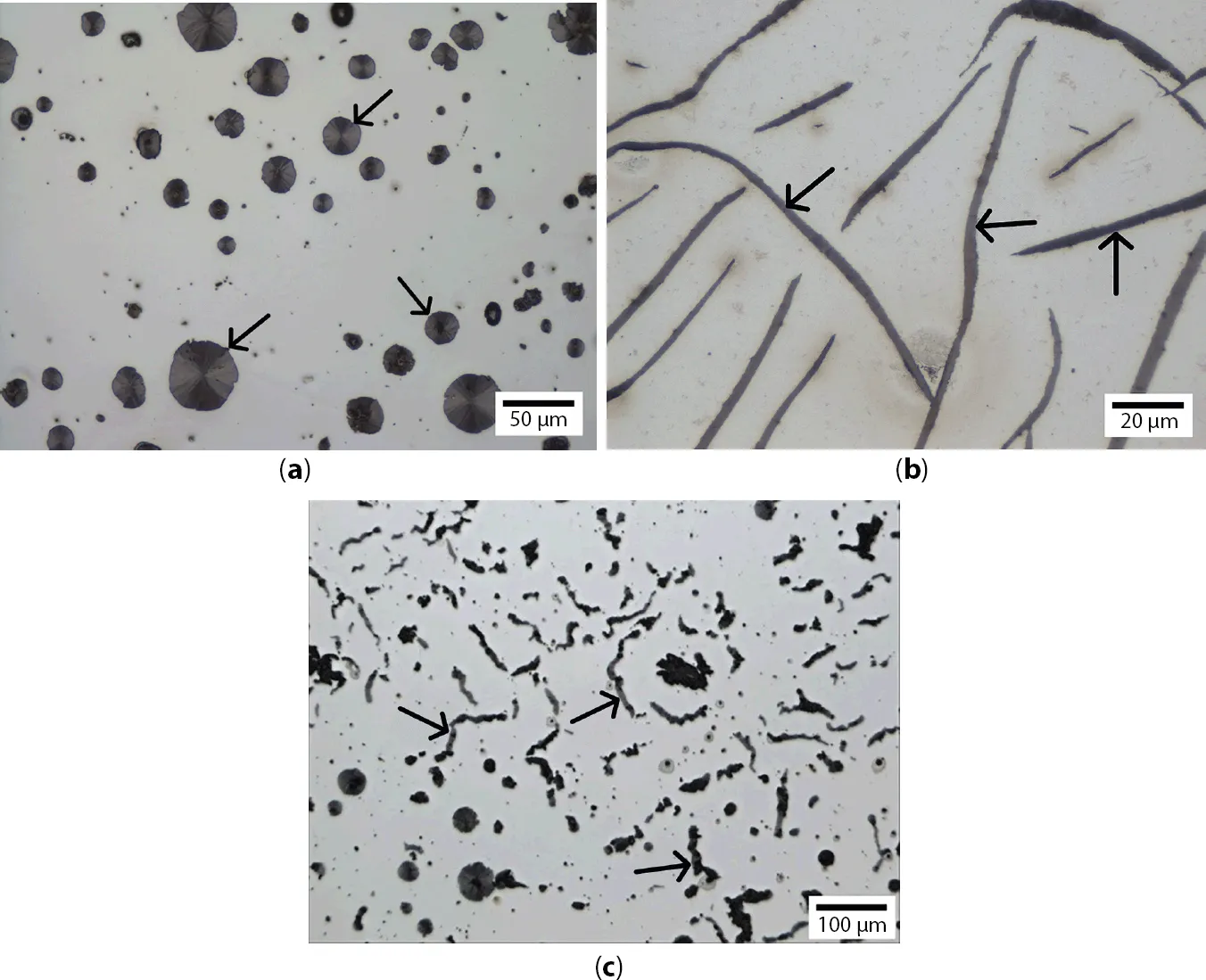
1.1.1 Spheroidal Graphite in Ductile Iron
Table of contents
- Cover
- Title Page
- Copyright
- Preface
- Chapter 1: Graphite in Metallic Materials Growths, Structures, and Defects of Spheroidal Graphite in Ductile Iron
- Chapter 2: Graphene—Synthesis and Quality Optimization
- Chapter 3: Methods of Synthesis and Physicochemical Properties of Fluorographenes
- Chapter 4: Graphene–SiC Reinforced Hybrid Composite Foam: Response to High Strain Rate Deformation
- Chapter 5: Atomic Structure and Electronic Properties of Few-Layer Graphene on SiC(001)
- Chapter 6: Features and Prospects for Epitaxial Graphene on SiC
- Chapter 7: Graphitic Carbon/Graphene on Si(111) via Direct Deposition of Solid-State Carbon Atoms: Growth Mechanism and Film Characterization
- Chapter 8: Chemical Reactivity and Variation in Electronic Properties of Graphene on Ni(111) and Reduced Graphene Oxide
- Chapter 9: Chlorophyll and Graphene: A New Paradigm of Biomimetic Symphony
- Chapter 10: Graphene Structures: From Preparations to Applications
- Chapter 11: Three-Dimensional Graphene-Based Structures: Production Methods, Properties, and Applications
- Chapter 12: Electrochemistry of Graphene Materials
- Chapter 13: Hydrogen Functionalized Graphene Nanostructure Material for Spintronic Application
- Chapter 14: The Impact of Uniaxial Strain and Defect Pattern on Magnetoelectronic and Transport Properties of Graphene
- Chapter 15: Exploiting Graphene as an Efficient Catalytic Template for Organic Transformations: Synthesis, Characterization and Activity Evaluation of Graphene-Based Catalysts
- Chapter 16: Exfoliated Graphene-Based 2D Materials: Synthesis and Catalytic Behaviors
- Chapter 17: Functionalization of Graphene with Molecules and/or Nanoparticles for Advanced Applications
- Chapter 18: Carbon Allotropes, Between Diamond and Graphite: How to Create Semiconductor Properties in Graphene and Related Structures
- Index
- End User License Agreement