
Chemistry for Pharmacy Students
General, Organic and Natural Product Chemistry
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Chemistry for Pharmacy Students
General, Organic and Natural Product Chemistry
About this book
Introduces the key areas of chemistry required for all pharmacy degree courses and focuses on the properties and actions of drug molecules
This new edition provides a clear and comprehensive overview of the various areas of general, organic, and natural products chemistry (in relation to drug molecules). Structured to enhance student understanding, it places great emphasis on the applications of key theoretical aspects of chemistry required by all pharmacy and pharmaceutical science students. This second edition particularly caters for the chemistry requirements in any 'Integrated Pharmacy Curricula', where science in general is meant to be taught 'not in isolation', but together with, and as a part of, other practice and clinical elements of the course.
Chemistry for Pharmacy Students: General, Organic and Natural Product Chemistry, 2nd Edition is divided into eight chapters. It opens with an overview of the general aspects of chemistry and their importance to modern life, with emphasis on medicinal applications. The text then moves on to discuss the concepts of atomic structure and bonding and the fundamentals of stereochemistry and their significance to pharmacy in relation to drug action and toxicity. Various aspects of organic functional groups, organic reactions, heterocyclic chemistry, nucleic acids and their pharmaceutical importance are then covered in subsequent chapters, with the final chapter dealing with drug discovery and development, and natural product chemistry.
- Provides a student-friendly introduction to the main areas of chemistry required by pharmacy degree courses
- Written at a level suitable for non-chemistry students in pharmacy, but also relevant to those in life sciences, food science, and the health sciences
- Includes learning objectives at the beginning of each chapter
- Focuses on the physical properties and actions of drug molecules
Chemistry for Pharmacy Students: General, Organic and Natural Product Chemistry, 2nd Edition is an essential book for pharmacy undergraduate students, and a helpful resource for those studying other subject areas within pharmaceutical sciences, biomedical sciences, cosmetic science, food sciences, and health and life sciences.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
Chapter 1
Introduction
Learning Objectives
- describe the role of chemistry in modern life;
- define some of the physical properties of drugs, for example, melting point, boiling point, polarity, solubility and acid‐base properties;
- explain the terms pH, pK a, buffer and neutralization.
1.1 ROLE OF CHEMISTRY IN MODERN LIFE
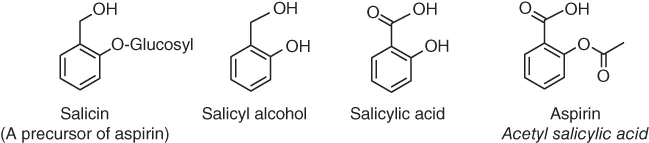
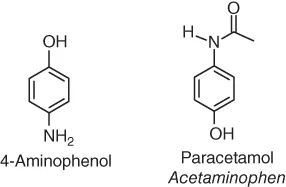
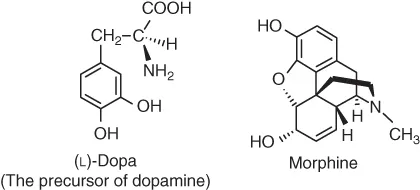
Table of contents
- COVER
- TABLE OF CONTENTS
- PREFACE TO THE SECOND EDITION
- PREFACE TO THE FIRST EDITION
- CHAPTER 1: INTRODUCTION
- CHAPTER 2: ATOMIC STRUCTURE AND BONDING
- CHAPTER 3: STEREOCHEMISTRY
- CHAPTER 4: ORGANIC FUNCTIONALGROUPS
- CHAPTER 5: ORGANIC REACTIONS
- CHAPTER 6: HETEROCYCLICCOMPOUNDS
- CHAPTER 7: NUCLEIC ACIDS
- CHAPTER 8: NATURAL PRODUCTCHEMISTRY
- Index
- END USER LICENSE AGREEMENT