
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
Compiling all the information available on the topic, this ready reference covers all important aspects of iron oxides.
Following a preliminary overview chapter discussing iron oxide minerals along with their unique structures and properties, the text goes on to deal with the formation and transformation of iron oxides, covering geological, synthetic, and biological formation, as well as various physicochemical aspects. Subsequent chapters are devoted to characterization techniques, with a special focus on X-ray-based methods, magnetic measurements, and electron microscopy alongside such traditional methods as IR/Raman and Mossbauer spectroscopy. The final section mainly concerns exciting new applications of magnetic iron oxides, for example in medicine as microswimmers or as water filtration systems, while more conventional uses as pigments or in biology for magnetoreception illustrate the full potential.
A must-read for anyone working in the field.
Following a preliminary overview chapter discussing iron oxide minerals along with their unique structures and properties, the text goes on to deal with the formation and transformation of iron oxides, covering geological, synthetic, and biological formation, as well as various physicochemical aspects. Subsequent chapters are devoted to characterization techniques, with a special focus on X-ray-based methods, magnetic measurements, and electron microscopy alongside such traditional methods as IR/Raman and Mossbauer spectroscopy. The final section mainly concerns exciting new applications of magnetic iron oxides, for example in medicine as microswimmers or as water filtration systems, while more conventional uses as pigments or in biology for magnetoreception illustrate the full potential.
A must-read for anyone working in the field.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Iron Oxides by Damien Faivre in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Inorganic Chemistry. We have over one million books available in our catalogue for you to explore.
Information
Chapter 1
Introduction
Damien Faivre
1.1 Iron Oxides: From Nature to Applications
As the name of the book “Iron oxides: from nature to applications” suggests, iron oxides are not only widespread in the environment, but also widely used by mankind in a variety of applications (Figure 1.1). Both this ubiquitous presence in nature and the utilization as tools have been established for centuries and are still valid today. The first illustrative examples of iron oxides certainly are compass needle or rust (Figure 1.2). Iron oxides are present in solid, liquid, and gaseous environments, with respective examples such as rocks, as mineral inclusion in swimming bacteria or in aerosols. Depending on the type of use, several sources of iron oxides exist. Applications range from the heavy steel production to medicine and art. The different aspects of mineral formation and their use as well as modern characterization techniques are reviewed in this book.
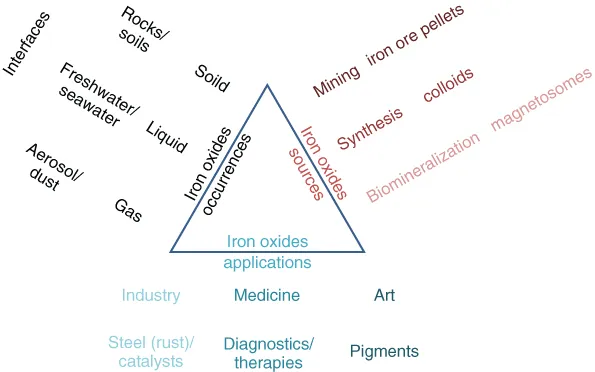
Figure 1.1 Scheme of the iron oxide occurrences, sources, and applications.

Figure 1.2 Images of agricultural machine left in a field for decades (a). A closer view clearly shows the presence of rust (b).
As a consequence of this omnipresence and significance in scientific and technological fields, a multidisciplinary interest has emerged with iron oxides at the center of its focus (Figure 1.3). The books in the collection by Cornell and Schwertmann were the most recent examples of efforts to summarize the knowledge on the subject [1–3]. Here, we focus on scientific aspects that have developed in the meantime and are therefore mostly not present in the book series published more than a decade ago. We also present some topics that were simply not addressed previously. This is particularly true for biological iron oxide formation, the role of which has only recently been recognized, as well as for the application aspects related to the iron oxides, which were not in the focus of the previous books.
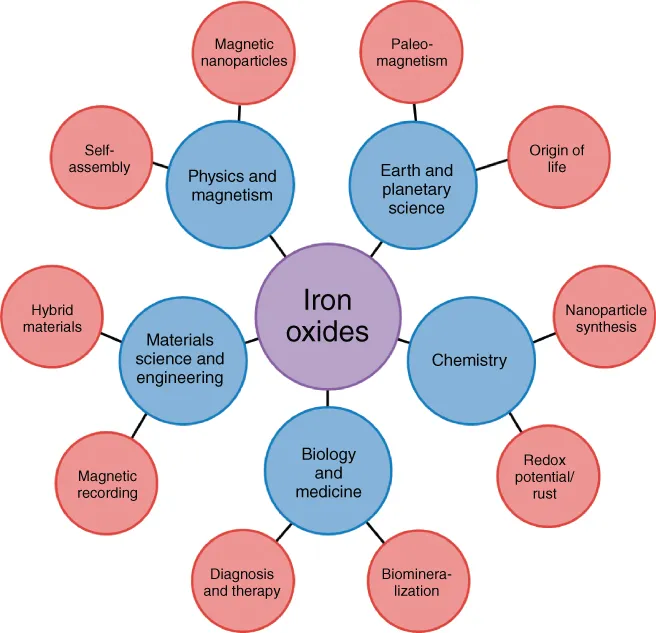
Figure 1.3 The iron oxides at the core of a multidisciplinary interest.
1.2 A Very Brief Overview of the Iron Oxides and How They Found Names
There are 16 iron oxides, hydroxides, or oxyhydroxides recognized so far, all called in short iron oxides (Table 1.1). Most of them were discovered and described at the beginning of the nineteenth century. In the table below, the compounds are simply classified based on their composition, that is, they are made from ferric, ferrous, or ferric and ferrous iron; and contain oxides (“O”), hydroxides (“OH”), or oxides and hydroxides.
Table 1.1 Summary of the different known iron oxides
| Iron oxides | Iron oxyhydroxides | Iron hydroxides | |
| Fe(II) compounds | Wüstite FeO [4, 5] | “White rust” – Fe(OH)2 [6, 7] | |
| Fe(II)-Fe(III) compounds | Magnetite Fe3O4 [8] | “Green rusts” – Fougèrite [Fe2+4Fe3+2(OH)12][CO3]·3H2O [9] | |
| Fe(III) compounds | Hematite α-Fe2O3 [8] | Goethite α-FeOOH [8] | Bernalite Fe(OH)3 [10] |
| β-Fe2O3 [11] | Akaganéite β-FeOOH [12] | ||
| Maghemite γ-Fe2O3 [13] | Lepidocrocite γ-FeOOH [14] | ||
| δ-Fe2O3 [15] | Feroxyhyte δ-FeOOH [16–18] | ||
| ϵ-Fe2O3 [19] | |||
| Ferrihydrite 5Fe2O3·9H2O [20, 21] | |||
| Schwertmannite Fe8O8(OH)6(SO4)·nH2O [8, 22] |
The references to the minerals are discussed in the text since some mineral names have varied over time.
With the advancement of the characterization and synthesis techniques, some of them were only named or fully characterized after lively debates. For example, the first mineral liste...
Table of contents
- Cover
- Related Titles
- Title Page
- Copyright
- Table of Contents
- List of Contributors
- Foreword
- Preface
- Chapter 1: Introduction
- Part I: Formation, Transformation
- Part II: Characterization Techniques
- Part III: Applications
- Index
- End User License Agreement