
- English
- ePUB (mobile friendly)
- Available on iOS & Android
About this book
Provides a comprehensive, yet practical source of reference, and excellent foundation for comparing the properties and performance of coatings and selecting the most suitable materials based on specific service needs and environmental factors.
Coating technology has developed significant techniques for protecting existing infrastructure from corrosion and erosion, maintaining and enhancing the performance of equipment, and provided novel functions such as smart coatings greatly benefiting the medical device, energy, automotive and construction industries.
The mechanisms, usage, and manipulation of cutting-edge coating methods are the focus of this book. Not only are the working mechanisms of coating materials explored in great detail, but also craft designs for further optimization of more uniform, safe, stable, and scalable coatings.
A group of leading experts in different coating technologies demonstrate their main applications, identify the key bottlenecks, and outline future prospects. Advanced Coating Materials broadly covers the coating techniques, including cold spray, plasma vapor deposition, chemical vapor deposition, sol–gel method, etc., and their significant applications in microreactor technology, super(de)wetting, joint implants, electrocatalyst, etc. Numerous kinds of coating structures are addressed, including nanosize particles, biomimicry structures, metals and complexed materials, along with the environmental and human compatible biopolymers resulting from microbial activities. This state-of-the-art book is divided into three parts: (1) Materials and Methods: Design and Fabrication, (2) Coating Materials: Nanotechnology, and (3) Advanced Coating Technology and Applications.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Part I
MATERIALS AND METHODS: DESIGN AND FABRICATION
Chapter 1
The Science of Molecular Precursor Method
Abstracts
1.1 Metal Complex
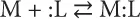
- Chemical reactions, in particular complex formation, can be classified as acid–base ones; the resulting products can be examined as complexes of the type Lewis acids and bases.
- All acids and bases can be divided into hard, soft, and/or intermediate.
- The HSAB principle itself is the following: the acid–base reactions take place in such a way that hard acids prefer to be connected with hard bases, meanwhile soft acids react with soft bases.
| Metal | Ligand | |
| Hard | H+, Li+, Na+, K+, Be2+, Mg2+, Ca2+, Sr2+, Mn2+, Al3+, N3+, As3+, Cr3+, Co3+, Fe3+, Si4+, Sn4+, BF3, AlCl3, CO2 | H2O, OH–, F–, SO42–, PO43–, CH3CO2–, RO–, Cl–, ClO4–, NO3–, ROH, NH3, RNH2 |
| Borderline | Fe2+, Co2+, Ni2+, Cu2+, Zn2+, Pb2+, Sn2+, Sb3+, Bi3+, Rh3+, Ir3+, SO2, NO+, Ru2+, Os2+, R3C+, C6H5+ | C6H5NH2, C5H5N, N3–, Br–, NO2–, SO32–, N2 |
| Soft | Ag+, Cu+, Au+, Tl+, Hg+, Pd2+, Cd2+, Pt2+, Hg2+, Pt4+, Tl3+, RS+, I+, HO+, I2, Br2, ICN, | R2S, RSH, RS–, I–, SCN–, R3P, CN–, RCN, CO, C2H4, C6H6, H– |
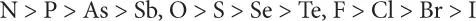
Table of contents
- Cover
- Title page
- Copyright page
- Preface
- Part I: Materials and Methods: Design and Fabrication
- Part II: Coating Materials Nanotechnology
- Part III: Advanced Coating Technology and Applications
- Index
- End User License Agreement
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app