![]()
Chapter 1
Particle Engineering of Polymers into Multifunctional Interactive Excipients
Sharad Mangal, Ian Larson, Felix Meiser and David AV Morton*
Monash Institute of Pharmaceutical Sciences, Monash University, Melbourne, Australia
Abstract
Both natural and man-made polymers are widely utilized as tablet binders and filler-binders. The physicochemical and mechanical properties such as particle size, shape and deformation behavior of polymeric binders are key in their effective use. Many such binders are applied as solution in a wet granulation process, which facilitate its facile distribution leading to improved effectiveness as a binder. Direct compression and dry granulation are recognized as routes with reduced process complexity and cost. These processes require a binder to be employed in a dry form and it can be more difficult to obtain a homogeneous distribution of a dry binder in a powder formulation. Therefore, these binders are required in high proportions to generate mechanically strong tablets. At lower proportions, they often are insufficient to create mechanically strong tablets. Recently, innovations in the generation of co-processed excipients have been proposed. Co-processing is a popular means of improving excipient functionalities, where two or more existing excipients are combined by some suitable means to generate new structures with improved and often combined functionalities as compared to the component excipients. Particle size reduction is known to improve the binder properties of an excipient, but also makes it highly cohesive and hard to blend. Via particle engineering, surface structure of smaller particles can be tailored to optimize the cohesive-adhesive balance (CAB) of the powder, allowing formation of interactive mixtures. This chapter reviews recent efforts to engineer surface-modified polymeric micro-excipient structures with the inherent ability to not only form an interactive mixture efficiently and provide flow enhancement, but also to create harder tablets at lower proportions. Hence, this approach represents a potential novel multifunctional prototype polymeric micro-excipient for direct compression and dry granulation processes.
Keywords: Particle engineering, powder technology, interactive mixtures, tablets, binder, multifunctional excipients
1.1 Introduction
The modern pharmaceutical market is under relentless pressure from slowing new product approvals, patent expiries and global competition. In addition, new opportunities exist with an evolving patient population, numerous unmet medical needs and growing disease awareness. The pharmaceutical industry must evolve and improve product developing and manufacturing efficiencies for sustainable performance. Efficient and cost-effective product development and manufacturing are continually being explored to meet the challenge of not only reducing cost but also reducing the risk of product recalls.
Tablets are the most commonly used pharmaceutical preparation, accounting for more than 80% of all dosage forms administered [1]. The principal reasons for their continued popularity include convenience of administration and patient preference, high-precision dosing, stability and cost effectiveness [2].
Tablets are typically manufactured by applying pressure to active pharmaceutical ingredient(s) (APIs) and excipients powder blends in a die using a punch, which compresses the powder into a coherent compact. Under compression, bonds are established between the particles, thus conferring a certain mechanical strength to the compact. A formulation must exhibit good flow and high compactability for an API to be transformed into tablets of satisfactory quality. Good flow is necessary to ascertain the rapid and reproducible filling of powder into the die to minimize weight variation; while high compactability is required to ensure that the tablets are sufficiently strong to withstand handling during manufacturing and transportation [3].
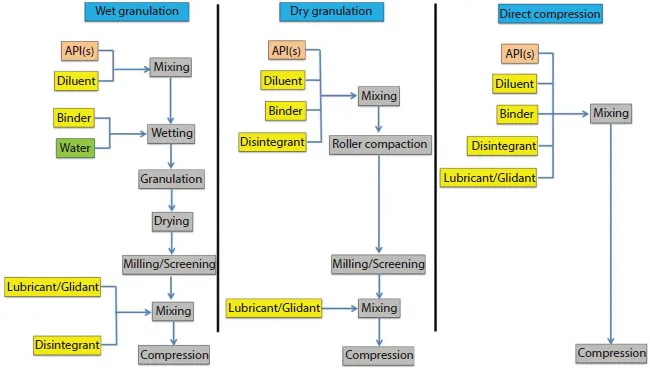
The majority of API(s) lack the requisite flow and compactability for direct tablet manufacturing [4]. Therefore, the flow and compactability of the API(s) need to be adjusted to ensure formation of high-quality tablets. Typically, the flow and compactability of a tablet formulation is improved by a granulation step (wet or dry granulation) in which the particles of API(s) and excipients are agglomerated into larger particulate structures referred to as granules. Wet granulation of the input materials can improve the flow properties for further processing and can create non-segregating blends of powder ingredients [5]. However, it involves multiple manufacturing steps, which can add significant time and cost to the process. Conversely, direct compression merely involves mixing of API(s) and excipients followed by immediate compression (Figure 1.1). Therefore, direct compression is an attractive manufacturing process, with fewer steps, for reducing cost and improving manufacturing output.
1.2 Polymers as Excipients
Excipients form an integral part of any pharmaceutical tablet formulation. They play the fundamental role in creation of robust tablet formulations by carrying out an extensive range of functions such as fillers, binders, disintegrants, lubricants, glidants, coating agent and anti-adherents. Currently, a wide range of polymeric materials are used as excipients [6,7], and polymers are the largest overall consumed product segment for the global excipients market, accounting for over 30% [8]. The excipient market is expected to grow at an annual rate of 5.2% from 2013 to 2018, to reach around $7.35 billion by 2018 [8].
Polymers of natural, semi-synthetic and synthetic origin are used especially in the role of binder and filler-binder (see Table 1.1). Polymeric excipients are popular as they can be tailored for many applications by altering their chain length and by chemical functionalization. This can achieve new materials with various optimized physicochemical and mechanical properties for such specific applications.
Table 1.1 List of polymeric excipients, their source and functionalities. This table is compiled from the information given in the Handbook of Pharmaceutical Excipients [9].
| Polymeric Excipient | Source | Functionality |
| Natural |
| Zein | Extracted from corn gluten | Binder, Coating agent |
| Cellulose | Extracted from fibrous plant material | Diluent, Disintegrant |
| Alginic acid | Extracted from various species of brown seaweed | Binder, Disintegrant |
| Acacia | Exudate from the stems and branches of Acacia Senegal | Binder |
| Guar gum | Extracted from the endosperm of the Cyamopsis tetragonolobus | Binder, Disintegrant |
| Inulin | Extracted from the tubers of Dahlia variabilis, Helianthus | Binder |
| Chitosan | Extracted from shells of crustaceans such as shrimps and crabs | Binder, Coating agent |
| Semi-synthetic |
| Sodium alginate | By neutralized alginic acid with sodium bicarbonate | Binder, Disintegrant |
| Calcium alginate | By treating sodium alginate with calcium salts | Disintegrant |
| Methyl cellulose | By treating wood pulp with alkali followed by methylation | Binder, Disintegrant, Coating agent |
| Carboxymethyl cellulose sodium | By treating wood pulp with alkali followed by reaction with sodium monochloroacetate | Binder, Disintegrant |
| Carboxymethyl cellulose calcium | By treating wood pulp with alkali followed by methylation and then converting to calcium salt | Disintegrant |
| Cellulose acetate | By treating cellulose with acid catalysis and acetic anhydride | Diluent, Coating agent |
| Cellulose acetate phthalate | By reacting cellulose acetate with phthalic anhydride | Coating agent |
| Microcrystalline cellulose | By controlled hydrolysis of cellulose with mineral acid | Binder, Diluent, Disintegrant |
| Hydroxypropylmethyl cellulose | By treating alkali cellulose with chloromethane and propylene oxide | Binder, Coating agent |
| Hydroxypropylmethyl cellulose acetate succinate | By the esterification of hydroxypropylmethyl cellulose with acetic anhydride and succinic anhydride | Film coating, Enteric coating |
| Hydroxypropylmethyl cellulose phthalate | By the esterification of hydroxypropylmethyl cellulose with phthalic anhydride | Enteric coating |
| Ethylcellulose | By ethylation of the alkali cellulose with chloroethane | Binder, Diluent, Coating agent |
| Low substituted-hydroxypropyl cellulose | By reacting alkaline cellulose with propylene oxide | Binder, Disintegrant |
| Ethyl cellulose | By ethylation of the alkali cellulose with chloroethane | Binder, Diluent, Coating a... |