
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
Providing a thorough overview of leading research from internationally-recognized contributing authors, this book describes methods for the preparation and application of redox systems for organic electronic materials like transistors, photovoltaics, and batteries.
- Covers bond formation and cleavage, supramolecular systems, molecular design, and synthesis and properties
- Addresses preparative methods, unique structural features, physical properties, and material applications of redox active p-conjugated systems
- Offers a useful guide for both academic and industrial chemists involved with organic electronic materials
- Focuses on the transition-metal-free redox systems composed of organic and organo main group compounds
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Organic Redox Systems by Tohru Nishinaga in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Organic Chemistry. We have over one million books available in our catalogue for you to explore.
Information
1
INTRODUCTION: BASIC CONCEPTS AND A BRIEF HISTORY OF ORGANIC REDOX SYSTEMS
Tohru Nishinaga
Department of Chemistry, Graduate School of Science and Engineering, Tokyo Metropolitan University, Hachioji, Tokyo, Japan
1.1 REDOX REACTION OF ORGANIC MOLECULES
Redox is a portmanteau word of “reduction” and “oxidation.” Originally, oxidation meant a chemical reaction in which oxygen combines with another substance, after Antoine Lavoisier, late in the eighteenth century, called a product of the reaction an oxide [1]. The term “reduction” had been used long before the introduction of the term “oxidation” in the smelting to produce iron from ore and coke [1]. In the contemporary definition recommended by IUPAC [2], oxidation is a reaction that satisfies criteria 1 “the complete, net removal of one or more electrons from a molecular entity” and 2 “an increase in the oxidation number of any atom within any substrate” and meets in many cases criterion 3 “gain of oxygen and/or loss of hydrogen of an organic substrate.” Conversely, reduction is the reverse process of oxidation.
For transition metals, a direct one-electron transfer related to the aforementioned criterion 1 is common due to their relatively lower ionization energy in comparison with main group elements [3] and low reactivity of the unpaired d-electrons. In contrast, the mechanisms of common organic redox reactions do not involve a direct one-electron transfer [4], and reactions based on the criterion 3 are typical. For example, oxidation of primary alcohol (RCH2OH) to aldehyde (RHC═O) with Cr(VI)O3 proceeds via chromic ester intermediate (RCH2O3Cr(VI)OH), and proton and HOCr(IV)O2− are eliminated from the intermediate [5] (Scheme 1.1a). In this reaction, the total number of electrons in the outer shell decreases from 14 at the C─O moiety to 12 at the C═O moiety, that is, two-electron oxidation, while the formal oxidation number of Cr changes from +6 to +4, that is, two-electron reduction. Similarly, reduction of carbonyl group to alcohol with NaBH4 in ethanol formally proceeds via nucleophilic attack of a pair of electrons in hydride to electron-deficient carbonyl carbon [5] (Scheme 1.1b). Thus, formally, a pair of two electrons moves together in typical organic redox reactions as known in other organic reactions such as substitutions.
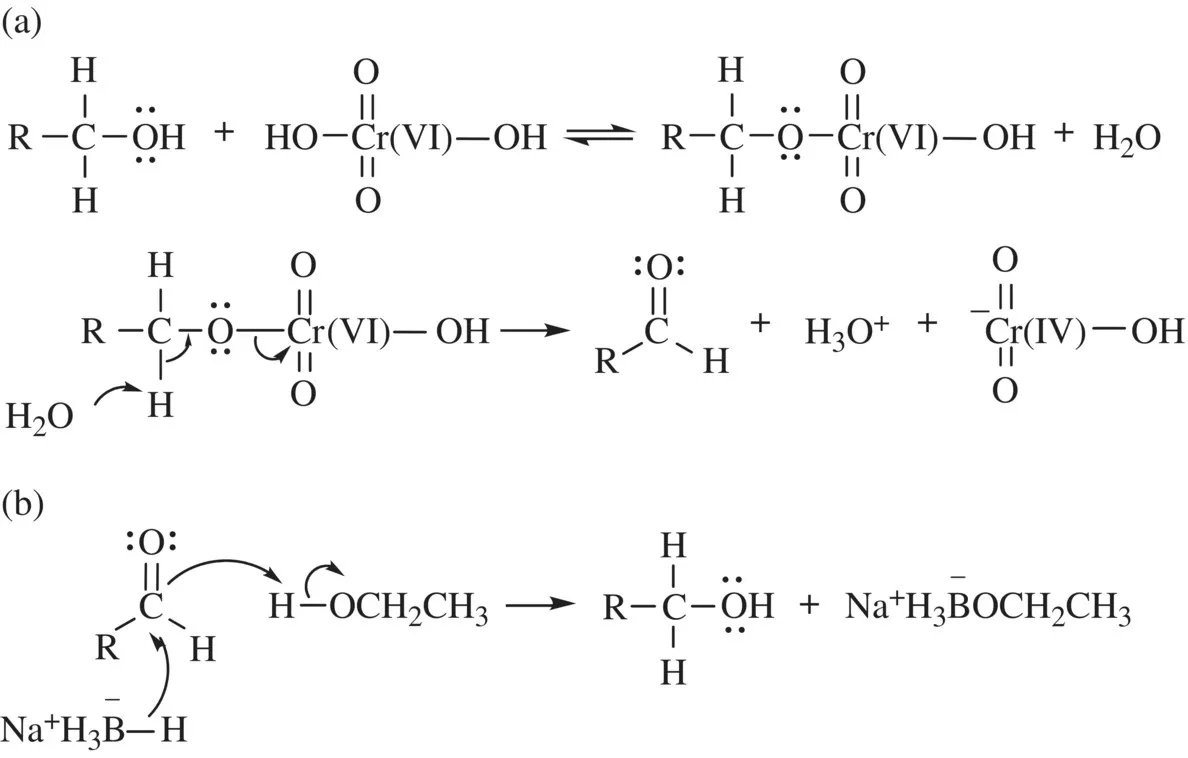
SCHEME 1.1 (a) Oxidation of alcohol to aldehyde with Cr(VI) and (b) hydride reduction of aldehyde to alcohol.
On the other hand, one-electron oxidation or reduction of a neutral or ionic molecule (Scheme 1.2) gives generally highly reactive ion radicals or radicals, and follow-up reactions such as radical coupling and deprotonation are prone to take place [6]. Nevertheless, some organic molecules give persistent species after one-electron transfer at ambient temperature [7, 8]. Simple π-extension and substituents of resonance electron donating R2N─, RO─, RS─ or withdrawing N≡C─, C═O groups cause delocalization of spin and charge density, which reduces the reactivity of the reactive center. As the other thermodynamic stabilization, aromatization after electron transfer plays an important role for certain molecules. An appropriate steric protection is also an effective strategy for protecting a reactive radical center [9]. As a result of these effects, they can be reversibly regenerated by the reverse electron transfer. This book deals with organic π-electron systems and related organo main group compounds that show such reversible one-electron transfer.
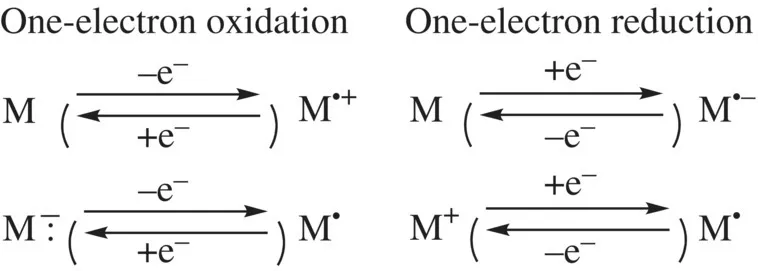
SCHEME 1.2 One-electron oxidation and reduction of neutral and ionic molecules.
1.2 REDOX POTENTIAL IN NONAQUEOUS SOLVENTS
Redox potential is the important measure for redox systems, by which one can predict how easily one-electron oxidation or reduction takes place with other redox reagents. For the measurement of redox potential, cyclic voltammetry is usually the first choice, because not only the redox potential but also the stability of the species generated after electron transfer can be observed. Several types of reference electrodes are used to measure redox potentials. The standard hydrogen electrode (SHE) or normal hydrogen electrode (NHE), which is deter...
Table of contents
- COVER
- TITLE PAGE
- TABLE OF CONTENTS
- LIST OF CONTRIBUTORS
- PREFACE
- 1 INTRODUCTION: BASIC CONCEPTS AND A BRIEF HISTORY OF ORGANIC REDOX SYSTEMS
- 2 REDOX-MEDIATED REVERSIBLE σ-BOND FORMATION/CLEAVAGE
- 3 REDOX-CONTROLLED INTRAMOLECULAR MOTIONS TRIGGERED BY π-DIMERIZATION AND PIMERIZATION PROCESSES
- 4 TETRATHIAFULVALENE: A REDOX UNIT FOR FUNCTIONAL MATERIALS AND A BUILDING BLOCK FOR SUPRAMOLECULAR SELF-ASSEMBLY
- 5 ROBUST AROMATIC CATION RADICALS AS REDOX TUNABLE OXIDANTS
- 6 AIR-STABLE REDOX-ACTIVE NEUTRAL RADICALS: TOPOLOGICAL SYMMETRY CONTROL OF ELECTRONIC-SPIN, MULTICENTERED CHEMICAL BONDING, AND ORGANIC BATTERY APPLICATION
- 7 TRIARYLAMINE-BASED ORGANIC MIXED-VALENCE COMPOUNDS: THE ROLE OF THE BRIDGE
- 8 MAGNETIC PROPERTIES OF MULTIRADICALS BASED ON TRIARYLAMINE RADICAL CATIONS
- 9 OPEN-SHELL π-CONJUGATED HYDROCARBONS
- 10 INDENOFLUORENES AND RELATED STRUCTURES
- 11 THIENOACENES
- 12 CATIONIC OLIGOTHIOPHENES: p-DOPED POLYTHIOPHENE MODELS AND APPLICATIONS
- 13 ELECTRON-DEFICIENT CONJUGATED HETEROAROMATICS
- 14 OLIGOFURANS
- 15 OLIGOPYRROLES AND RELATED COMPOUNDS
- 16 PHOSPHOLES AND RELATED COMPOUNDS: SYNTHESES, REDOX PROPERTIES, AND APPLICATIONS TO ORGANIC ELECTRONIC DEVICES
- 17 ELECTROCHEMICAL BEHAVIOR AND REDOX CHEMISTRY OF BOROLES
- 18 ISOLATION AND CRYSTALLIZATION OF RADICAL CATIONS BY WEAKLY COORDINATING ANIONS
- 19 HEAVIER GROUP 14 ELEMENT REDOX SYSTEMS
- 20 π-ELECTRON REDOX SYSTEMS OF HEAVIER GROUP 15 ELEMENTS
- INDEX
- END USER LICENSE AGREEMENT