
Process Modeling and Simulation for Chemical Engineers
Theory and Practice
- English
- ePUB (mobile friendly)
- Available on iOS & Android
About this book
This book provides a rigorous treatment of the fundamental concepts and techniques involved in process modeling and simulation. The book allows the reader to:
(i) Get a solid grasp of "under-the-hood" mathematical results
(ii) Develop models of sophisticated processes
(iii) Transform models to different geometries and domains as appropriate
(iv) Utilize various model simplification techniques
(v) Learn simple and effective computational methods for model simulation
(vi) Intensify the effectiveness of their research
Modeling and Simulation for Chemical Engineers: Theory and Practice begins with an introduction to the terminology of process modeling and simulation. Chapters 2 and 3 cover fundamental and constitutive relations, while Chapter 4 on model formulation builds on these relations. Chapters 5 and 6 introduce the advanced techniques of model transformation and simplification. Chapter 7 deals with model simulation, and the final chapter reviews important mathematical concepts.
Presented in a methodical, systematic way, this book is suitable as a self-study guide or as a graduate reference, and includes examples, schematics and diagrams to enrich understanding. End of chapter problems with solutions and computer software available online at www.wiley.com/go/upreti/pms_for_chemical_engineers are designed to further stimulate readers to apply the newly learned concepts.Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
Chapter 1
Introduction
1.1 System
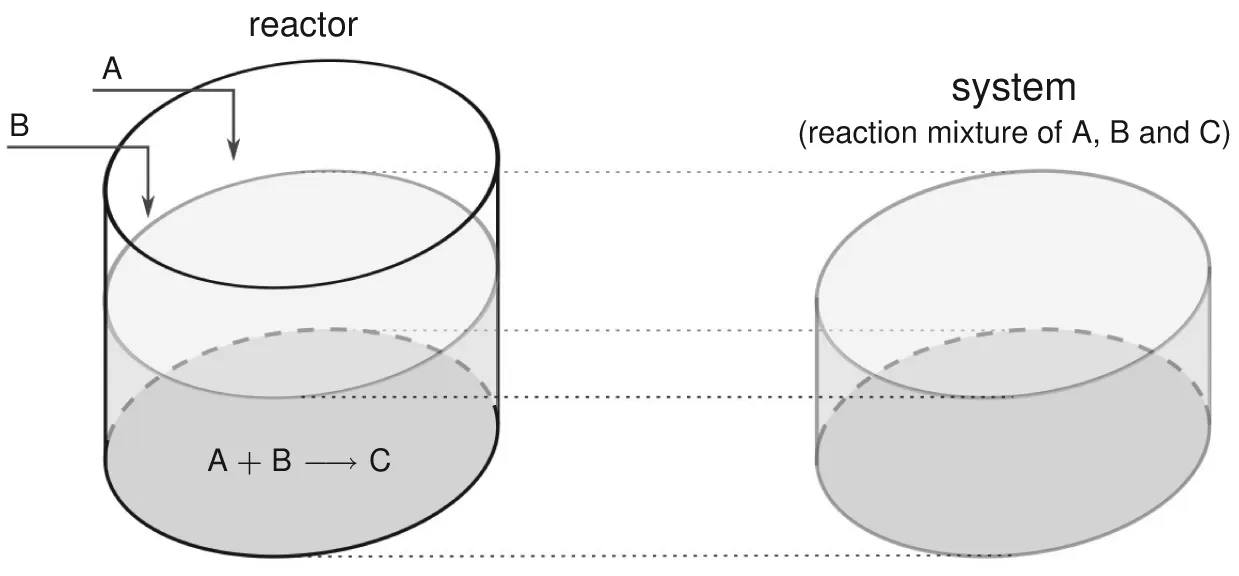
1.1.1 Uniform System
1.1.2 Properties of System
- the same value of concentration of the species A throughout the system (or uniform concentration of A),
- uniform concentration of each of the remaining species B and C, and
- a similar uniformity of any other intensive property, e.g., temperature.
Table of contents
- Cover
- Title Page
- Copyright
- Dedication
- Table of Contents
- Preface
- Notation
- Chapter 1: Introduction
- Chapter 2: Fundamental Relations
- Chapter 3: Constitutive Relations
- Chapter 4: Model Formulation
- Chapter 5: Model Transformation
- Chapter 6: Model Simplification and Approximation
- Chapter 7: Process Simulation
- Chapter 8: Mathematical Review
- Index
- End User License Agreement