
eBook - ePub
Natural Products Targeting Clinically Relevant Enzymes
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Natural Products Targeting Clinically Relevant Enzymes
About this book
The past decade has seen the reappearance of natural products as a valuable source of potent therapeutics. Here, experts on bioactive natural products cover the full spectrum of clinically relevant enzymes that are known to be targeted by natural products. Key enzymes include acetylcholine esterase, angiotensin-I-converting enzyme, cyclooxygenase, dihydrofolate reductase, phospholipase A2, respiratory complexes, and many more.
By connecting the diversity of medicinal natural product sources with their potential clinical applications, this volume serves as a companion for the medicinal chemist looking for innovative small molecule compounds as well as for pharmacologist interested in the clinical effects and mode of action of herbal and traditional medicines.
By connecting the diversity of medicinal natural product sources with their potential clinical applications, this volume serves as a companion for the medicinal chemist looking for innovative small molecule compounds as well as for pharmacologist interested in the clinical effects and mode of action of herbal and traditional medicines.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Natural Products Targeting Clinically Relevant Enzymes by Paula B. Andrade,Patrícia Valentão,David M. Pereira in PDF and/or ePUB format, as well as other popular books in Médecine & Pharmacologie. We have over one million books available in our catalogue for you to explore.
Information
Chapter 1
Natural Products as Enzyme Inhibitors
David M. Pereira, Catarina Andrade, Patrícia Valentão and Paula B. Andrade
REQUIMTE/LAQV, Universidade do Porto, Laboratório de Farmacognosia Departamento de Química, Faculdade de Farmácia, Rua de Jorge Viterbo Ferreira, Nº 228, 4050-213, Porto, Portugal
1.1 Why Are Natural Products Good Enzyme Inhibitors?
Natural products are widely distributed and their unique properties have been explored for centuries by our earliest ancestors to treat diseases and injuries. Throughout evolution, the potential of natural products as modulators of biological functions has been increasingly realized [1].
Over the past decades, there has been a decrease in the use of natural products by pharmaceutical companies as a starting point for drug discovery, essentially due to the belief that natural products were somehow incompatible with drug discovery approaches that were based on high-throughput screening directed towards molecular targets [2]. Furthermore, there was also the assumption that combinatorial chemistry techniques would be able to generate all the chemical diversity needed for successful lead discovery. However, the results of many large combinatorial screening collections have proved to be quite discouraging and it has already been recognized that diversity within biologically relevant ‘chemical space’ is more important than library size. To a certain point, libraries of synthetic molecules have been designed to mimic the chemical properties of the natural compounds [3].
Despite the deficiency of investment in natural products as main leads in drug discovery over the past decades, 34% of the medicines approved by the US Food and Drug Administration (FDA) between 1981 and 2010 were actually natural products or directly derived from them [4].
The great potential of molecules of natural origin in drug discovery arises from their remarkable chemical and structural diversity. About 40% of the chemical scaffolds found in natural products are indeed absent in today’s medicinal chemistry synthetic libraries. For this reason, the use of nature-inspired molecules is a good complement to synthetically produced molecules [5, 6].
One of the most relevant reasons for the success of natural products as a source of bioactive molecules arises from their ‘drug-likeness’, which frequently surpasses that of synthetic compounds. Considering their biosynthetic processes in living organisms, it is not surprising that natural molecules display greater similarity and binding potential with biological structures, thus increasing the probability of an effective interaction with different biological targets [6].
One of the most outstanding features of natural products is their three-dimensional conformation, which is attributed to the complex and unique structure that is mostly beyond the synthetic capacity of medicinal chemistry. Natural products are often described for their ‘privileged’ scaffold, allowing them to work as ligands for a diverse array of enzymes and receptors. This term, first mentioned by Evans in the late 1980s, was originally used to address the benzodiazepines scaffold, privileged by their ability to bind not only to their receptors at the central and peripheral nervous system, but also to cholecystokinin receptors. In this way, and according to Evans’s definition, a privileged structure displays affinity to several receptors/proteins [7, 8]. In 2010, Matthew et al., presented an exhaustive review by providing a comprehensive list of privileged scaffolds found in both synthetic drugs and natural ones. Spiket-p, integramycin and routiennocin, despite having the same scaffold (6,6-spiroacetal), display different bioactivities and are found in different species, which demonstrates the evolution-driven predisposition for repetition, once a suitable solution to a particular biochemical problem has been found (Figure 1.1). This can also explain the non-random patterning of macromolecular structures in living systems. Consequently, 6,6-spiroacetal is a ‘privileged’ scaffold found in a number of natural products displaying the ability to bind to different targets thereby exerting different pharmacological effects [9].
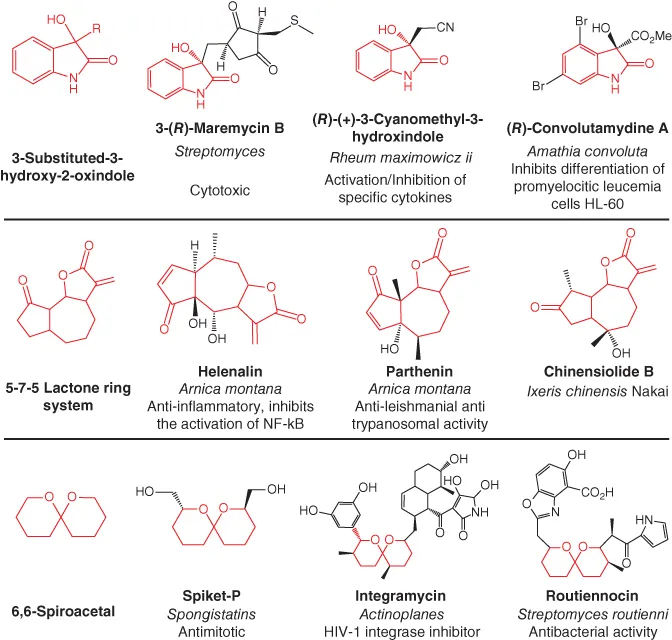
Figure 1.1 Examples of ‘privileged’ scaffolds found in natural products.
Adapted from [9].)
Considering the enormous variety of compounds occupying the ‘chemical space’, it can easily be assumed that natural products cover distinct regions when compared with synthetic ones, having wider and more drug-like properties. Rosén et al. demonstrated throughout computational screenings that natural products cover parts of the chemical space that lack representation by medicinal chemistry compounds and, by doing so, these compounds may be useful for novel leads [10].
For obvious reasons, the difference between natural products and other sources of molecules, with relevance to their ability to display biological properties, has a chemical basis [11, 12]. In general, the composition of natural molecules is distinct from that of synthetic ones, as they display fewer nitrogen, sulfur or halogen atoms, being richer in oxygen and containing more hydrogen bond donors. The sterical complexity of natural products also plays a role in this equation, these molecules presenting a larger number of rings and, overall, more chiral centres.
But why? Why do natural products present such chemical traits? The obvious answer is that they are not randomly synthesized, instead resulting from biosynthetic processes that are highly targeted. For this reason, these molecules are meant to interact with molecular targets that are, themselves, three-dimensional and chiral. In addition, the enzymes involved in the biosynthesis are usually chiral in the way they usually yield a single isomer, a trait not always found in medicinal chemistry, where racemic mixtures are frequently produced [12]. Enzymes involved in their natural biosynthesis, as well as the molecular targets the natural product is meant to interact with, are inherently three-dimensional and chiral as human enzymes are. Thus, there is actually a link that can explain why natural products can display good results as enzyme inhibitors [12].
The fact that the majority of natural products exhibits such characteristics, shows that they result from an evolutionary drive that selects molecules displaying a certain arrangement of atoms [12, 13]. In addition, the probability of finding a bioactive molecule is much higher in natural products when compared with a randomly synthesized molecule [8]. This is not surprising if we consider that it arises from nature’s own high-throughput screening: not only are molecules prone to display the well-defined three-dimensional structure described above, but they are also produced to target well-conserved biological targets in a certain mechanism of action, meaning that they are synthesized to display some kind of activity towards a biological target [9, 14].
Another striking difference between natural products and randomly synthesized molecules rests in the underlying synthesis strategy in both cases. Unlike combinatorial chemistry, which can make use of tens of thousands of different scaffolds as building blocks, no such mechanism is available in nature. In fact, for bi...
Table of contents
- Cover
- Title Page
- Copyright
- Table of Contents
- List of Contributors
- Chapter 1: Natural Products as Enzyme Inhibitors
- Chapter 2: Molecular Targets of Clinically Relevant Natural Products from Filamentous Marine Cyanobacteria
- Chapter 3: Natural Angiotensin Converting Enzyme (ACE) Inhibitors with Antihypertensive Properties
- Chapter 4: Phospholipase A2 Inhibitors of Marine Origin
- Chapter 5: β-Secretase (BACE1) Inhibitors from Natural Products
- Chapter 6: Hypoglycaemic Effects of Plants Food Constituents via Inhibition of Carbohydrate-Hydrolysing Enzymes: From Chemistry to Future Applications
- Chapter 7: Natural Products Targeting Clinically Relevant Enzymes of Eicosanoid Biosynthesis Implicated in Inflammation and Cancer
- Chapter 8: Anti-HIV Natural Products
- Chapter 9: Natural Inhibitors of Mitochondrial Respiratory Chain: Therapeutic and Toxicological Implications
- Chapter 10: Targeting Enzymatic Pathways with Marine-Derived Clinical Agents
- Chapter 11: Anti-Malarial Drug Discovery: New Enzyme Inhibitors
- Chapter 12: Natural Plant-Derived Acetylcholinesterase Inhibitors: Relevance for Alzheimer’s Disease
- Index
- End User License Agreement