- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Process Scale Purification of Antibodies
About this book
Promoting a continued and much-needed renaissance in biopharmaceutical manufacturing, this book covers the different strategies and assembles top-tier technology experts to address the challenges of antibody purification. • Updates existing topics and adds new ones that include purification of antibodies produced in novel production systems, novel separation technologies, novel antibody formats and alternative scaffolds, and strategies for ton-scale manufacturing
• Presents new and updated discussions of different purification technologies, focusing on how they can address the capacity crunch in antibody purification
• Emphasizes antibodies and innovative chromatography methods for processing
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Process Scale Purification of Antibodies by Uwe Gottschalk in PDF and/or ePUB format, as well as other popular books in Medicine & Pharmacology. We have over one million books available in our catalogue for you to explore.
Information
1
DOWNSTREAM PROCESSING OF MONOCLONAL ANTIBODIES: CURRENT PRACTICES AND FUTURE OPPORTUNITIES
Brian Kelley
Bioprocess Development, Genentech, Inc., South San Francisco, CA, USA
1.1 INTRODUCTION
Monoclonal antibodies (mAbs) are established as the most prevalent class of recombinant protein therapeutics. They can be expressed at high levels in cell culture, are typically highly soluble, and are relatively stable during processing. The nearly universal use of mammalian cell expression systems for mAb synthesis, combined with the selection of homologous, humanized mAb framework protein sequences, provides opportunities to harmonize manufacturing processes around base platforms that can then be used with only slight variations from product to product. In addition, by using a platform process, manufacturing plants designed for the production of one mAb can usually be readily adapted to produce others.
For these reasons, mAbs represent a unique group of biological products. They accommodate rapid process development timelines, can be produced in large quantities, and may be manufactured in multiple facilities during their product life cycle. As a result, they have relatively low manufacturing costs and benefit from the flexibility of production at either in‐house or contract production facilities. Although mAbs are not commodity products that can be substituted in the clinical setting, they have distinct advantages in production scale and cost, as well as in product development speed and convenience, when compared with other recombinant protein therapeutics.
This introductory chapter attempts to set the context for the following chapters, which cover many aspects of mAb purification in detail. A typical mAb purification process flowsheet is described and used to illustrate the impact of purification platforms on mAb production. Factors to consider with respect to the various process alternatives or new technologies described in later chapters are addressed, emphasizing the integration of unit operations and process design principles into an optimized, holistic process. Both current practices and controversial topics are introduced, among them the challenges of very‐large‐scale (VLS) production, issues related to facility fit, the maturation of separation technology for mAb processing, the need for innovations in purification technology, and the impact of the evolving regulatory environment. It is hoped that this backdrop will stimulate critical thinking and comprehensive analysis when the processing options described in the following chapters are being considered.
1.2 A BRIEF HISTORY OF CURRENT GOOD MANUFACTURING PROCESS mAb AND INTRAVENOUS IMMUNOGLOBULIN PURIFICATION
The processes used for production of intravenous immunoglobulin (IVIG) from human plasma differ from those used for recombinant mAbs. Figure 1.1 shows a consensus processing scheme, based on many published process flowsheets, for the purification of IVIG. Most IVIG processes lack chromatography steps and instead rely on multiple fractional precipitation steps based on the Cohn process [1]. Some recently developed processes include chromatography, but this is used to a limited extent and still in combination with upstream steps based on the Cohn process [2, 3]. The processes used for recombinant mAb purification have borrowed very little from plasma fractionation technology, other than ultrafiltration to formulate and concentrate the drug substance. The low cost of manufacturing IVIG and the very large production scale have led to debate on the value of going “back to the future” and applying IVIG processing technologies to the production of recombinant mAbs. A review of current mAb processing platforms will put this proposal into context.
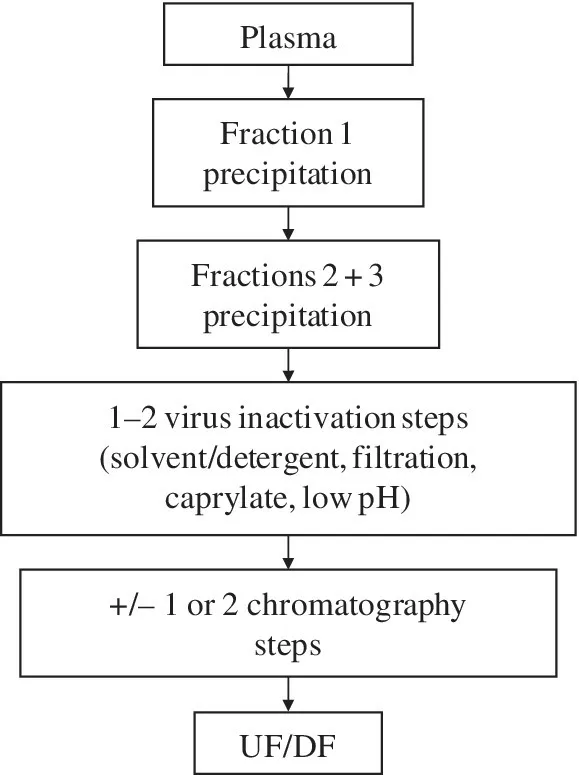
FIGURE 1.1 Cohn‐based IVIG consensus manufacturing process. UF/DF, ultrafiltration/diafiltration.
The first current good manufacturing process (cGMP) for mAb purification reflected the state of the art in the 1980s and early 1990s, prior to the accumulation of substantial process knowledge and the introduction of improved separations media that made today’s more efficient and scalable processes possible. Examples of the diversity of early processes include the use of various microfiltration or depth‐filtration media for harvest; affinity chromatography with Protein G in addition to Protein A; conventional capture columns to protect the Protein A resin; the incorporation of challenging separation methods for large‐scale production, such as size‐exclusion chromatography (SEC); solvent/detergent virus‐inactivation methods; and the requirement for four or even more chromatography steps (Figure 1.2). In addition, downstream processing was sometimes performed in the cold. Chromatography media often provided relatively low loading capacities, which did not raise significant issues when cell culture titers were measured in hundreds of milligrams per liter. To address the need for kilogram‐scale production, very large bioreactors were used. The focus of capture resin selection was based on maximizing volumetric productivity and the ability to process large volumes of feed rapidly rather than the handling of large batches (greater than 20 kg of product). Many of these early mAb products were also derived from a more diverse set of framework protein sequences, reflecting the historical progression from murine and chimeric mAbs to today’s fully humanized antibodies, which gave rise to a more varied set of process flowsheets.
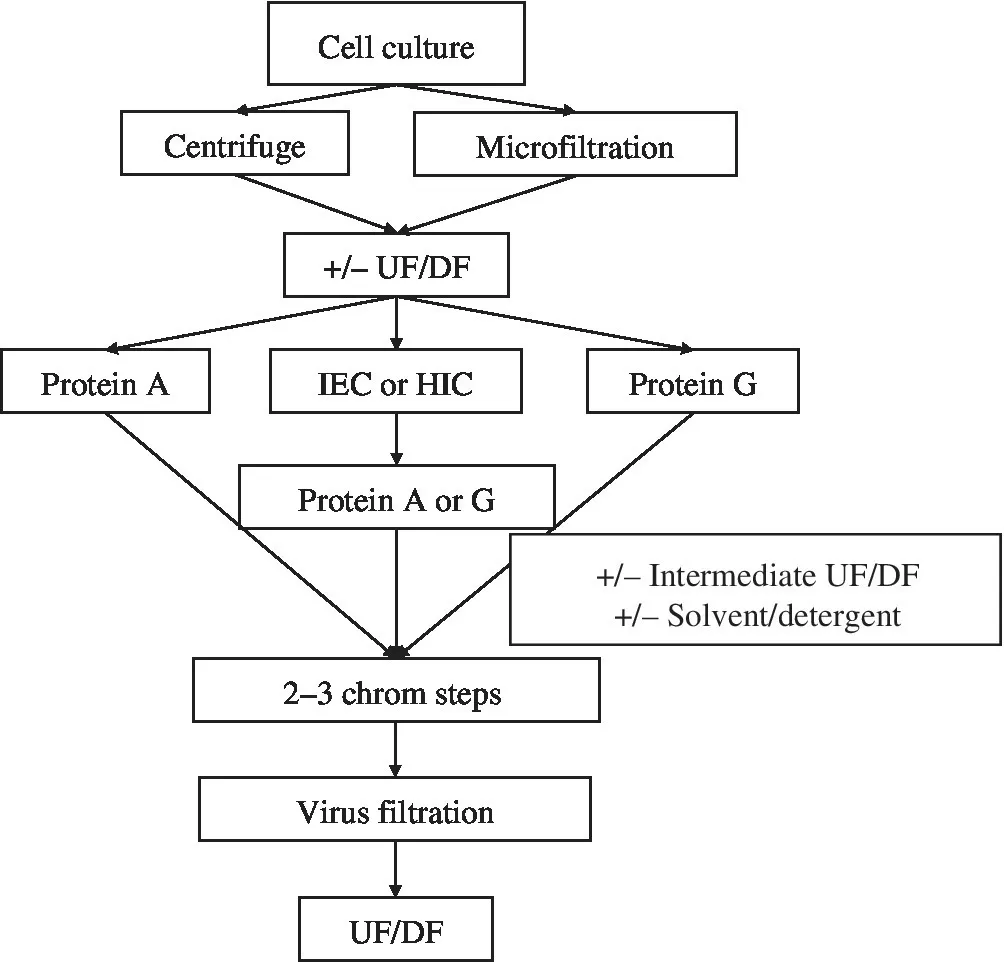
FIGURE 1.2 Early mAb purification schemes. HIC, hydrophobic interaction chromatography; IEX, ion‐exchange chromatography; UF/DF, ultrafiltration/diafiltration.
1.3 CURRENT APPROACHES IN PURIFICATION PROCESS DEVELOPMENT: IMPACT OF PLATFORM PROCESSES
Despite the extensive homology among humanized mAbs, variations in complementarity‐determining regions and framework sequences make it difficult to define a truly generic purification process capable of processing many different mAbs without any changes to the operating conditions. Many companies have defined platform purification processes based on a common sequence of unit operations. A frequently used purification platform for mAbs is shown in Figure 1.3. The conditioned medium is first clarified by centrifugation, followed by depth filtration. Protein A chromatography allows direct product capture from the centrate and achieves excellent purification and significant concentration of the product. The low‐pH elution from the Protein A step also accomplishes virus inactivation. Two chromatographic polishing steps are used to reduce host cell/medium‐derived and purification process‐related impurities. Additional virus removal is usually achieved in these polishing steps. One of the polishing steps is almost invariably anion‐exchange (AEX) chromatography, often in flow‐through mode. The second polishing step is typically cation‐exchange (CEX) chromatography, although occasionally ceramic hydroxyapatite chromatography and hydrophobic interaction chromatography (HIC) are used. The remaining process steps include virus removal filtration (VRF) and ultrafiltration/diafiltration (UF/DF) to formulate and concentrate the product, which is now the bulk drug substance. The efficiency, robustness, and scalability of this standardized process resulted in the rapid convergence of process development groups in the industry around this process flowsheet [4, 5]. Recently, several companies have introduced another step to this platform by adding detergent to the process pool to enable virus inactivation [6]. A properly designed step, and the choice of a nondenaturing detergent, may justify a claim of complete inactivation for enveloped viruses (typically a log reduction value (LRV) greater than six) without any product loss or quality impact. Alternatively, inactivation by arginine at neutral pH is suitable in lieu of detergent [7]. These robust in‐solution inactivation steps reduce the burden on the downstream chromatography steps and contribute to the overall viral safety claims, thus providing a significant advance in the field of process virology.
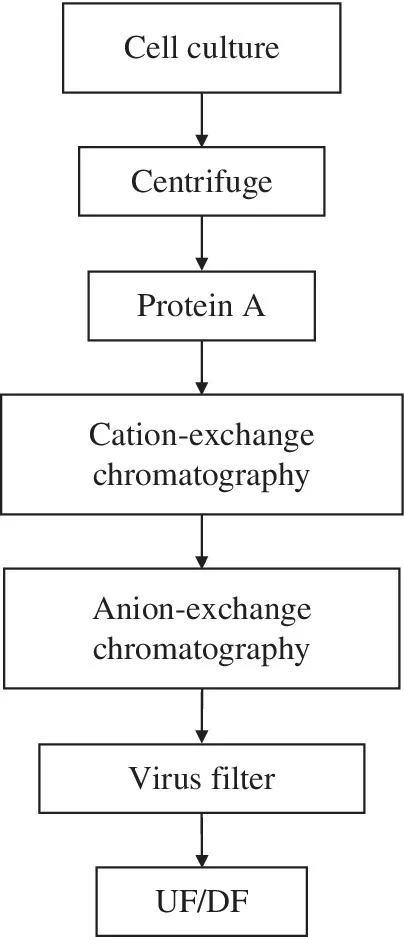
FIGURE 1.3 Typical current mAb production platform. UF/DF, ultrafiltration/diafiltration.
The establishment of platform processes for mAb production has already had an enormous impact on process development strategies and activities, and now also affects the world of commercial manufacturing. At this point, few companies have three or more commercial mAbs that are purified using a common platform process. Many mAbs currently in the clinical pipeline, however, are manufactured by a process similar to the standard process shown in Figure 1.3. The gradual progression of these early‐stage processes from clinical to commercial production enables additional efficiencies in production that will reduce the cost of goods (COGs) and accelerate responses to surges in product demand. The benefits of efficient facility management (e.g., reductions in changeover time and the use of common raw materials and equipment) and flexible commercial production (e.g., balanced production schedules among multiproduct facilities) will be realized more slowly than the gains seen today in the early stages of clinical development. The combination of platform processing, multiproduct facilities, rapid product changeover, and flexible sourcing between contract manufacturing organizations (CMOs) and in‐house production facilities achieves an industrialization of mAb production that will be unprecedented in the field of recombinant protein biologics. Antibodies could become a class of therapeutic biologics that support the treatment of large patient populations while remaining cost‐competitive with small molecules. To achieve this vision, the biopharmaceutical industry must take advantage of the opportunities presented by the ease of development, validation, and production afforded by platform processes.
Given the value and broad adoption of processing platforms, combined with an installed production facility base designed for them, there is enormous pressure to conform to such platforms with future products. As a result, there are limited options for unit operations, raw materials, step sequences, control systems and algorithms, and processing equipment. Although these restrictions may at first seem highly constraining, they require other challenges to be addressed, for example, the establishment of highly efficient work processes that rapidly define the appropriate processing conditions for each new mAb that enters the pipeline, as well as the definition of a common set of optimization approaches and process‐characterization studies that will streamline late‐stage clinical development.
Clarification operations such as centrifugation often vary little from product to product, provided that the cell culture process is not radically different. Large changes in the density or viability of cells in a bioreactor will affect clarification, but provided that the unit operations are designed for the worst‐case feed stream, few if any modifications will be needed for new mAbs. The capacity of centrate depth filters can vary significantly, depending on the feed stream, and should be optimized for robustness while the costs of raw materials are minimized. Similarly, the platform’s UF steps (VRF and final UF) should be largely unaffected by the change in the mAb. The unit operations that are most likely to require tuning are the chromatography steps. Even there, the standardization of many elements in a chromatography unit operation will streamline development timelines by focusing on key factors influenced by product characteristics [5]. Process variables that are often specified for platform processes include resin and membrane selection, column bed height, wash volumes, loading capacity, membrane flux, and target bulk concentration. This effort simplifies and accelerates early‐stage process development.
The Protein A capture step is generally a robust operation that can tolerate changes in bioreactor harvest conditions, cell culture titer, and product characteristics (see Chapter 5). The variables that may be influenced by product or feed stream variations are dynamic binding capacity, the optimal composition of the column wash solution, and the elution conditions. Variations in these process parameters arise from differences in the affinity of Protein A for the mAb, steric hindrance among molecules [8], and variations in impurity levels and species in the feed stream, probably caused either by the cell line and bioreactor management or by the properties of the mAb itself.
The most common variables for the ion‐exchange (IEX) polishing steps include the column‐loading and solution compositions (e.g., pH and counterion concentration) and the wash and elution compositions. In some cases, there can be major changes in the platform, for example, when a highly acidic mAb has strong affinity for an AEX resin and the typical flow‐through operation must be abandoned...
Table of contents
- COVER
- TITLE PAGE
- TABLE OF CONTENTS
- PREFACE
- LIST OF CONTRIBUTORS
- 1 DOWNSTREAM PROCESSING OF MONOCLONAL ANTIBODIES
- 2 THE DEVELOPMENT OF ANTIBODY PURIFICATION TECHNOLOGIES
- 3 HARVEST AND RECOVERY OF MONOCLONAL ANTIBODIES
- 4 NEXT-GENERATION CLARIFICATION TECHNOLOGIES FOR THE DOWNSTREAM PROCESSING OF ANTIBODIES
- 5 PROTEIN A-BASED AFFINITY CHROMATOGRAPHY
- 6 PURIFICATION OF HUMAN MONOCLONAL ANTIBODIES
- 7 HYDROPHOBIC INTERACTION CHROMATOGRAPHY FOR THE PURIFICATION OF ANTIBODIES
- 8 PURIFICATION OF MONOCLONAL ANTIBODIES BY MIXED-MODE CHROMATOGRAPHY
- 9 ADVANCES IN TECHNOLOGY AND PROCESS DEVELOPMENT FOR INDUSTRIAL-SCALE MONOCLONAL ANTIBODY PURIFICATION
- 10 ALTERNATIVES TO PACKED-BED CHROMATOGRAPHY FOR ANTIBODY EXTRACTION AND PURIFICATION
- 11 PROCESS-SCALE PRECIPITATION OF IMPURITIES IN MAMMALIAN CELL CULTURE BROTH
- 12 CHARGED ULTRAFILTRATION AND MICROFILTRATION MEMBRANES FOR ANTIBODY PURIFICATION
- 13 DISPOSABLE PREPACKED-BED CHROMATOGRAPHY FOR DOWNSTREAM PURIFICATION
- 14 INTEGRATED POLISHING STEPS FOR MONOCLONAL ANTIBODY PURIFICATION
- 15 ORTHOGONAL VIRUS CLEARANCE APPLICATIONS IN MONOCLONAL ANTIBODY PRODUCTION*
- 16 DEVELOPMENT OF A PLATFORM PROCESS FOR THE PURIFICATION OF THERAPEUTIC MONOCLONAL ANTIBODIES
- 17 THE EVOLUTION OF PLATFORM TECHNOLOGIES FOR THE DOWNSTREAM PROCESSING OF ANTIBODIES
- 18 COUNTERCURRENT CHROMATOGRAPHY FOR THE PURIFICATION OF MONOCLONAL ANTIBODIES, BISPECIFIC ANTIBODIES, AND ANTIBODY–DRUG CONJUGATES
- 19 THE EVOLUTION OF CONTINUOUS CHROMATOGRAPHY
- 20 ACCELERATED SEAMLESS ANTIBODY PURIFICATION
- 21 PROCESS ECONOMIC DRIVERS IN INDUSTRIAL MONOCLONAL ANTIBODY MANUFACTURE
- 22 DESIGN AND OPTIMIZATION OF MANUFACTURING
- 23 SMART DESIGN FOR AN EFFICIENT FACILITY WITH A VALIDATED DISPOSABLE SYSTEM
- 24 HIGH-THROUGHPUT SCREENING AND MODELING TECHNOLOGIES FOR PROCESS DEVELOPMENT IN ANTIBODY PURIFICATION
- 25 DOWNSTREAM PROCESSING OF MONOCLONAL ANTIBODY FRAGMENTS
- 26 DOWNSTREAM PROCESSING OF Fc FUSION PROTEINS, BISPECIFIC ANTIBODIES, AND ANTIBODY–DRUG CONJUGATES
- 27 MANUFACTURING CONCEPTS FOR ANTIBODY–DRUG CONJUGATES
- 28 PURIFICATION OF IgM and IgA
- 29 PURIFICATION OF MONOCLONAL ANTIBODIES FROM PLANTS
- 30 VERY-LARGE-SCALE PRODUCTION OF MONOCLONAL ANTIBODIES IN PLANTS
- 31 TRENDS IN FORMULATION AND DRUG DELIVERY FOR ANTIBODIES
- 32 ANTIBODY PURIFICATION
- INDEX
- END USER LICENSE AGREEMENT