
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Pharmaceutical Analysis for Small Molecules
About this book
A comprehensive introduction for scientists engaged in new drug development, analysis, and approvals
Each year the pharmaceutical industry worldwide recruits thousands of recent science graduates—especially chemistry, analytical chemistry, pharmacy, and pharmaceutical majors—into its ranks. However, because of their limited background in pharmaceutical analysis most of those new recruits find making the transition from academia to industry very difficult. Designed to assist both recent graduates, as well as experienced chemists or scientists with limited regulatory, compendial or pharmaceutical analysis background, make that transition, Pharmaceutical Analysis for Small Molecules is a concise, yet comprehensive introduction to the drug development process and analysis of chemically synthesized, small molecule drugs. It features contributions by distinguished experts in the field, including editor and author, Dr. Behnam Davani, an analytical chemist with decades of technical management and teaching experience in compendial, regulatory, and industry.
This book provides an introduction to pharmaceutical analysis for small molecules (non-biologics) using commonly used techniques for drug characterization and performance tests. The driving force for industry to perform pharmaceutical analyses is submission of such data and supporting documents to regulatory bodies for drug approval in order to market their products. In addition, related required supporting studies including good laboratory/documentation practices including analytical instrument qualification are highlighted in this book.
Topics covered include:
- Drug Approval Process and Regulatory Requirements (private standards)
- Pharmacopeias and Compendial Approval Process(public standards)
- Common methods in pharmaceutical analysis (typically compendial)
- Common Calculations for assays and impurities and other specific tests
- Analytical Method Validation, Verification, Transfer
- Specifications including how to handle out of specification (OOS) and out of trend (OOT)
- Impurities including organic, inorganic, residual solvents and elemental impurities
- Good Documentation Practices for regulatory environment
- Management of Analytical Laboratories
- Analytical Instrument Qualifications including IQ, OQ, PQ and VQ
Due to global nature of pharmaceutical industry, other topics on both regulatory (ICH) and Compendial harmonization are also highlighted.
Pharmaceutical Analysis for Small Molecules is a valuable working resource for scientists directly or indirectly involved with the drug development process, including analytical chemists, pharmaceutical scientists, pharmacists, and quality control/quality assurance professionals. It also is an excellent text/reference for graduate students in analytical chemistry, pharmacy, pharmaceutical and regulatory sciences.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Chapter 1
Drug Approval Process and Regulatory Requirements
1.1 Introduction
- 1. Analytics in the discovery process (R&D) of pharmaceutical products
- 2. Analytics in the compliance of products to their standards in the marketplace
1.2 The Regulatory Process for New Drug Entity
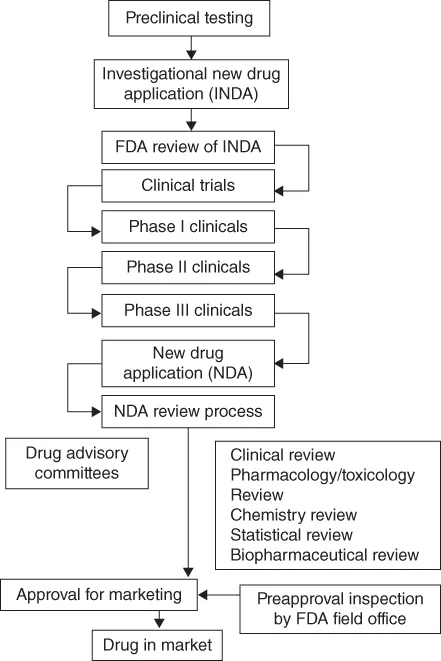
1.2.1 Preclinical Studies
1.2.2 Investigational New Drug Application (INDA)
1.2.2.1 Phase 1 Clinical
1.2.2.2 Phase 2 Clinical
1.2.2.3 Phase 3 Clinical
1.2.3 New Drug Application (NDA)
1.2.3.1 NDA Review by FDA
1.2.3.2 NDA Review Process
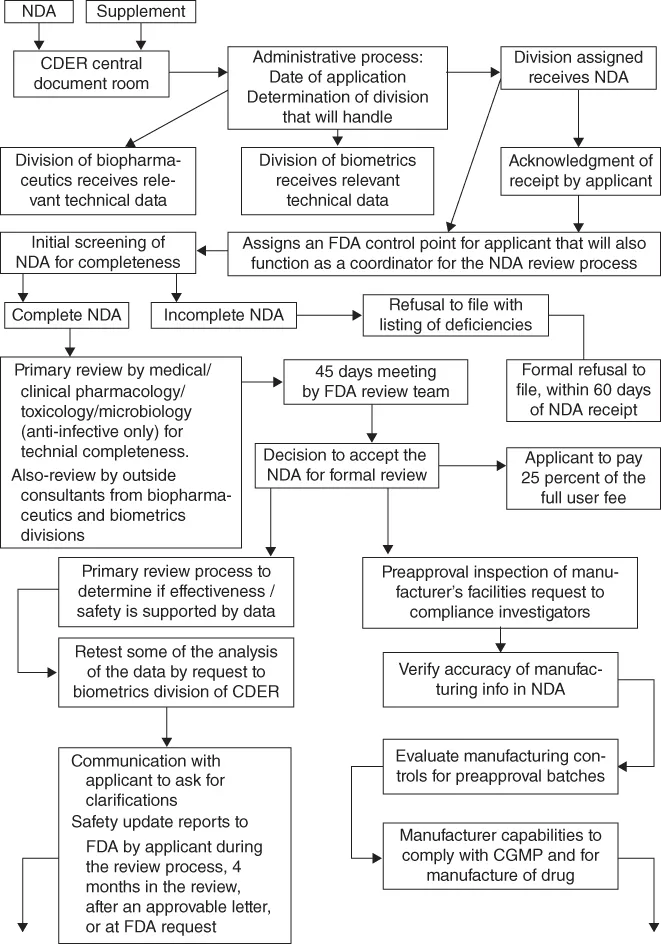
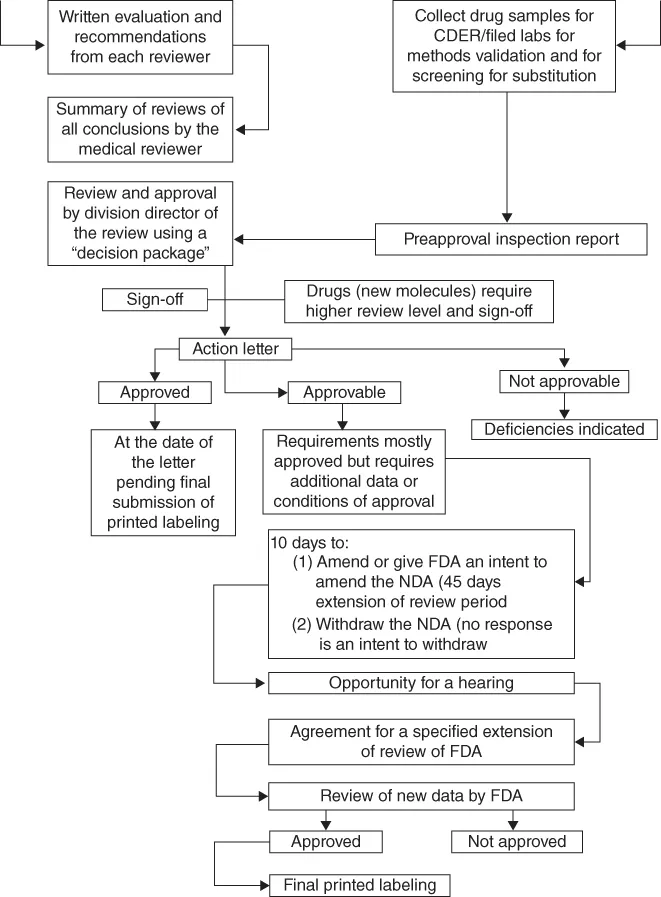
1.3 Good Laboratory Practice for Nonclinical Laboratory Studies
1.4 Validation of Analytical Procedures: Methodology
Table of contents
- Cover
- Title Page
- Copyright
- Dedication
- Table of Contents
- About the Editor
- List of Contributors
- Preface
- Acknowledgment
- Chapter 1: Drug Approval Process and Regulatory Requirements
- Chapter 2: Pharmacopeias and Compendial Approval Process
- Chapter 3: Common Methods in Pharmaceutical Analysis
- Chapter 4: Common Calculations
- Chapter 5: Analytical Method Validation, Verification, and Transfer
- Chapter 6: Specifications
- Chapter 7: Impurities
- Chapter 8: Good Documentation Practices
- Chapter 9: The Management of Analytical Laboratories
- Chapter 10: Analytical Instrument Qualification
- List of Abbreviations
- Index
- End User License Agreement
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app