
eBook - ePub
Enzymatic and Chemical Synthesis of Nucleic Acid Derivatives
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Enzymatic and Chemical Synthesis of Nucleic Acid Derivatives
About this book
A review of innovative tools for creative nucleic acid chemists that open the door to novel probes and therapeutic agents
Nucleic acids continue to gain importance as novel diagnostic and therapeutic agents. With contributions from noted scientists and scholars, Enzymatic and Chemical Synthesis of Nucleic Acid Derivatives is a practical reference that includes a wide range of approaches for the synthesis of designer nucleic acids and their derivatives.
The book covers enzymatic (including chemo-enzymatic) methods, with a focus on the synthesis and incorporation of modified nucleosides. The authors also offer a review of innovative approaches for the non-enzymatic chemical synthesis of nucleic acids and their analogs and derivatives, highlighting especially challenging species. The book offers a concise review of the methods that prepare novel and heavily modified polynucleotides in sufficient amount and purity for most clinical and research applications. This important book:
-Presents a timely and topical guide to the synthesis of designer nucleic acids and their derivatives
-Addresses the growing market for nucleotide-derived pharmaceuticals used as anti-infectives and chemotherapeutic agents, as well as fungicides and other agrochemicals.
-Covers novel methods and the most recent trends in the field
-Contains contributions from an international panel of noted scientistics
Written for biochemists, medicinal chemists, natural products chemists, organic chemists, and biotechnologists, Enzymatic and Chemical Synthesis of Nucleic Acid Derivatives is a practice-oriented guide that reviews innovative methods for the enzymatic as well as non-enzymatic synthesis of nucleic acid species.
Nucleic acids continue to gain importance as novel diagnostic and therapeutic agents. With contributions from noted scientists and scholars, Enzymatic and Chemical Synthesis of Nucleic Acid Derivatives is a practical reference that includes a wide range of approaches for the synthesis of designer nucleic acids and their derivatives.
The book covers enzymatic (including chemo-enzymatic) methods, with a focus on the synthesis and incorporation of modified nucleosides. The authors also offer a review of innovative approaches for the non-enzymatic chemical synthesis of nucleic acids and their analogs and derivatives, highlighting especially challenging species. The book offers a concise review of the methods that prepare novel and heavily modified polynucleotides in sufficient amount and purity for most clinical and research applications. This important book:
-Presents a timely and topical guide to the synthesis of designer nucleic acids and their derivatives
-Addresses the growing market for nucleotide-derived pharmaceuticals used as anti-infectives and chemotherapeutic agents, as well as fungicides and other agrochemicals.
-Covers novel methods and the most recent trends in the field
-Contains contributions from an international panel of noted scientistics
Written for biochemists, medicinal chemists, natural products chemists, organic chemists, and biotechnologists, Enzymatic and Chemical Synthesis of Nucleic Acid Derivatives is a practice-oriented guide that reviews innovative methods for the enzymatic as well as non-enzymatic synthesis of nucleic acid species.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Enzymatic and Chemical Synthesis of Nucleic Acid Derivatives by Jesús Fernández Lucas,María-José Camarasa Rius in PDF and/or ePUB format, as well as other popular books in Sciences biologiques & Biochimie. We have over one million books available in our catalogue for you to explore.
Information
1
Enzymatic Synthesis of Nucleoside Analogues by Nucleoside Phosphorylases
Sarah Kamel1,*, Heba Yehia1,2,*, Peter Neubauer1, and Anke Wagner1,3
1 Technische Universität Berlin, Department of Bioprocess Engineering, Institute of Biotechnology, Ackerstraße 76, 13355 Berlin, Germany
2 National Research Centre, Department of Chemistry of Natural Products, 12622 Giza, Egypt
3 BioNukleo GmbH, Ackerstraße 76, 13355 Berlin, Germany
1.1 Introduction
1.1.1 Nucleosides and Nucleoside Analogues
Nucleosides primarily consist of a nitrogenous base (nucleobase), which is either a purine base or a pyrimidine base and a five‐carbon sugar (pentose). The base and sugar are covalently linked via an N‐glycosidic bond (Figure 1.1). The pentose sugar moiety of naturally occurring canonical nucleosides is either ribose or deoxy‐ribose whereas the nucleobase might be either a purine (adenine, guanine) or a pyrimidine (cytosine, uracil, thymine). These nucleosides are structural subunits of nucleic acids and are involved in several cellular processes including enzyme regulation and metabolism, DNA and RNA synthesis, and cell signaling [1, 2].
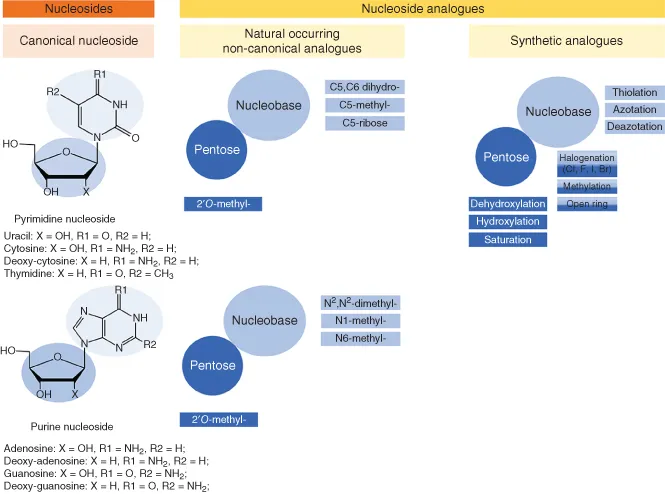
Figure 1.1 Classification of nucleosides and nucleoside analogues. Canonical (unmodified) nucleosides are the building blocks of DNA and RNA. Non‐canonical (naturally modified on pentose moiety, base moiety or both) are mainly occurring in RNA. Synthetic nucleosides are used in the treatment of viral and bacterial infections as well as in cancer treatment.
Naturally occurring nucleoside analogues (non‐canonical nucleosides) are found in almost all types of RNA especially in tRNAs and they are crucial for RNA processing. Non‐canonical analogues are nucleosides with different modifications on the pentose and/or the base [3] (Figure 1.1). There are more than 109 known post‐transcriptional modifications in the three phylogenetic domains [4]. Pseudouridine is the most ubiquitous analogue and is sometimes considered as the fifth RNA‐related nucleoside [5].
Non‐natural nucleoside analogues are synthetic molecules that structurally mimic their physiological counterparts and also act as antimetabolites [2]. Nucleoside analogues access cells through specific nucleoside transporters. Within the cells, they are phosphorylated by nucleoside kinases, which leads to increased levels of di‐ and tri‐phosphorylated nucleoside analogues in virus‐infected or cancer cells. The first and the second phosphorylation step can also be catalyzed by viral kinases in cells infected by some DNA viruses. Owing to differences in the substrate spectrum of human and viral kinases, virus‐specific drugs can be developed. The active forms of nucleoside analogues interfere with intracellular enzymes such as human and viral polymerases, kinases, DNA methyl transferase, ribonucleotide reductase, nucleoside phosphorylases (NPs) or thymidylate synthase [2, 6]. Furthermore, they can be incorporated into newly synthesized DNA and RNA, which may induce termination of the polymerization process, accumulation of mutations in viral progeny, or induction of apoptosis.
For more than 50 years, nucleosides and their analogues have been used as small molecule drugs for the treatment of several viral infections as well as for hematological malignancies and solid tumors. The first FDA approved antiviral nucleoside analogue was idoxuridine, which is used for the treatment of HSV‐1 (herpes simplex virus) [7]. In 1969, cytarabine was approved for the treatment of acute myeloid leukemia [2]. Since then, the interest in nucleoside analogues based drugs has tremendously grown. Currently, more than 39 approved nucleoside analogue drugs or drug combinations are approved for the treatment of seven human viral infections, which include HSV, varicella zoster virus (VZV), hepatitis‐B virus (HBV), hepatitis‐C virus (HCV), human immunodeficiency virus (HIV), respiratory syncytial virus (RSV), and human cytomegalovirus (HCMV) [7]. For treatment of cancer and viral infections, 50% and 20%, respectively, of all approved drugs belong to the class of nucleoside analogues [8]. Additional clinical indications for nucleoside analogues application include chronic hyperuricemia, immune suppression in organ transplant surgeries, and autoimmune disease as well as chronic obstructive pulmonary disease and asthma [2].
Emerging from the significance of nucleoside analogues, there have been continuous attempts to improve and simplify their synthesis processes. With the world moving toward green chemistry approaches, the enzymatic synthesis of nucleoside analogues offers several advantages over chemical methods, which include higher total yields, a higher regio‐ and stereo‐selectivity, and higher product purity. This allows for more biological and clinical trials [9]. Accordingly, enzymatic strategies are considered as a step forward to a more efficient synthesis of nucleosides and their analogues.
1.1.2 Enzymes Involved in the Enzymatic Synthesis of Nucleoside Analogues
Two main classes are employed in the enzymatic synthesis of nucleosides and their analogues: NPs and N‐deoxyribosyltransferases (NDTs). In this chapter, the focus is on enzymatic approaches using NPs. NPs are...
Table of contents
- Cover
- Table of Contents
- Preface
- 1 Enzymatic Synthesis of Nucleoside Analogues by Nucleoside Phosphorylases
- 2 Enzymatic Phosphorylation of Nucleosides
- 3 Enzymatic Synthesis of Nucleic Acid Derivatives Using Whole Cells
- 4 Enzymatic Synthesis of Nucleic Acid Derivatives by Immobilized Cells
- 5 Enzymatic Synthesis of Nucleic Acid Derivatives by Immobilized Enzymes
- 6 Synthesis of Nucleic Acid Derivatives by Multi‐Enzymatic Systems
- 7 Enzymatic Synthesis Using Polymerases of Modified Nucleic Acids and Genes
- 8 Synthetic Approaches to the Fleximer Class of Nucleosides – A Historic Perspective
- 9 Synthesis of Oligonucleotides Carrying Nucleic Acid Derivatives of Biomedical and Structural Interest
- 10 Synthesis of Carbohydrate–Oligonucleotide Conjugates and Their Applications
- 11 Advances in Light‐Directed Synthesis of High‐Density Microarrays and Extension to RNA and 2′F‐ANA Chemistries
- 12 SAMHD1‐Mediated Negative Regulation of Cellular dNTP Levels: HIV‐1, Innate Immunity, and Cancers
- Index
- End User License Agreement