
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
- Provides detailed methods to reduce or eliminate damage caused by corrosion
- Explains the human and environmental costs of corrosion
- Explains causes of and various types of corrosion
- Summarizes the costs of corrosion in different industries, including bridges, mining, petroleum refining, chemical, petrochemical, and pharmaceutical, pulp and paper, agricultural, food processing, electronics, home appliances etc
- Discusses the technical aspects of the various methods available to detect, prevent, and control corrosion
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Challenges in Corrosion by V. S. Sastri in PDF and/or ePUB format, as well as other popular books in Technology & Engineering & Materials Science. We have over one million books available in our catalogue for you to explore.
Information
CHAPTER 1
INTRODUCTION AND FORMS OF CORROSION
Corrosion is basically a combination of technical and economic problems. To understand the economics of corrosion, it is necessary that one is proficient in both the science of corrosion and the fundamental principles of economics. There are many forms of corrosion, which can be deleterious in a variety of ways. It is logical to discuss the various forms of corrosion of metallic structures occurring in different corrosive environments.
1.1 GENERAL OR UNIFORM OR QUASI-UNIFORM CORROSION
General corrosion is the most common form of corrosion. This can be uniform (even), quasi-uniform, or uneven. General corrosion accounts for the greatest loss of metal or material. Electrochemical general corrosion in aqueous media can include galvanic or bimetallic corrosion, atmospheric corrosion, stray current dissolution, and biological corrosion (Table 1.1).
Table 1.1 Forms of Corrosion1
| 1. General corrosion | Uniform, quasi-uniform, nonuniform corrosion, galvanic corrosion |
| 2. Localized corrosion | Pitting corrosion, crevice corrosion, filiform corrosion |
| 3. Metallurgically influenced corrosion | Intergranular corrosion, sensitization, exfoliation, dealloying |
| 4. Microbiologically influenced corrosion | |
| 5. Mechanically assisted corrosion | Wear corrosion, erosion–corrosion, corrosion fatigue |
| 6. Environmentally induced cracking | Stress-corrosion cracking; hydrogen damage, embrittlement; hydrogen-induced blistering; high-temperature hydrogen attack; hot cracking, hydride formation; liquid metal embrittlement; solid metal-induced embrittlement |
1 ASM Metals Handbook, Corrosion, Vol. 13, 9th ed., Craig and Pohlman, pp. 77–189.
Dissolution of steel or zinc in sulfuric or hydrochloric acid is a typical example of uniform electrochemical attack. Uniform corrosion often results from exposure to polluted industrial environments, exposure to fresh, brackish, and salt waters, or exposure to soils and chemicals. Some examples of uniform or general corrosion are the rusting of steel, the green patina on copper, tarnishing silver, and white rust on zinc on atmospheric exposure. Tarnishing of silver in air, oxidation of aluminum in air, attack of lead in sulfate-containing environments results in the formation of thin protective films and the metal surface remains smooth. Oxidation, sulfidation, carburization, hydrogen effects, and hot corrosion can be considered as types of general corrosion(16).
Liquid metals and molten salts at high temperatures lead to general corrosion(1). Microelectrochemical cells result in uniform general corrosion. Uniform general corrosion can be observed during chemical and electrochemical polishing and passivity where anodic and cathodic sites are physically inseparable. A polished surface of a pure active metal immersed in a natural medium (atmosphere) can suffer from galvanic cells. Most of the time, the asperities act as anodes and the cavities as cathodes. If these anodic and cathodic sites are mobile and change in a continuous dynamic manner, uniform or quasi-uniform corrosion is observed. If some anodic sites persist and are not covered by protective corrosion products, or do not passivate, localized corrosion is observed (1).
Some macroelectrochemical cells can cause a uniform or near-uniform general attack of certain regions. General uneven or quasi-uniform corrosion is observed in natural environments. In some cases, uniform corrosion produces a somewhat rough surface by the removal of a substantial amount of metal that either dissolves in the environment or reacts with it to produce a loosely adherent, porous coating of corrosion products. After careful removal of rust formed because of general atmospheric corrosion of steel, the surface reveals an undulated surface, indicating nonuniform attack of different areas (1) as shown in Figure 1.1.
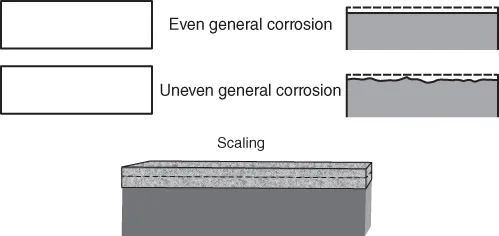
Figure 1.1 Even and uneven general corrosion and high-temperature attack. (Reproduced by permission, Elsevier Ltd. (2).)
In natural atmospheres, the general corrosion of metals can be localized. The corrosion morphology is dependent on the conductivity, ionic species, temperature of the electrolyte, alloy composition, phases, and homogeneity in the microstructure of the alloy, and differential oxygenation cell. The figure also shows high-temperature attack that is generally uniform. It is also possible to observe subsurface corrosion films within the matrix of the alloy because of the film formation at the interface of certain microstructures in several alloys at high temperatures (3).
The main factors governing general corrosion are: (i) agitation, (ii) pH of the medium, (iii) temperature, and (iv) protective passive films.
- The agitation of the medium has a profound influence on the corrosion performance of the metals as agitation accelerates corrosion performance of the metals, accelerates the diffusion of corrosive species, or destroys the passive film mechanically.
- Low pH (acidic) values accelerate the rate of corrosion as for an active metal such as iron or zinc, the cathodic reaction controls the rate of reaction in accordance with the equation
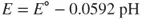 The plot of electrode potential against the logarithm of current density gives rise to a Tafel plot shown in Figure 1.2. From this plot, a logarithm of corrosion current density can be obtained. The Evans diagrams obtained by the extrapolation of Tafel slopes for the cathodic and anodic polarization curves shown in Figure 1.2 can also been seen in Figures 1.3 and 1.4. In general, the cathodic Tafel slopes are reproducible and reliable for evaluation of corrosion rates as they represent noncorroded original surface of the metal. It is obvious that the corrosion current is greater in acidic solution. The influence of pH also depends on the composition of the alloy as seen in Figure 1.4. When the zinc is present with mercury amalgam, the corrosion current is lower than when the metal is zinc alone. When zinc is present along with platinum, high corrosion rates are observed as platinum provides effective cathodic sites for hydrogen evolution. In addition to this, the stability of the passive film in acid, neutral, or alkaline pH is a contributing factor. Some examples are the stability of magnesium fluoride in alkaline medium and the amphoteric nature of aluminum oxide in pH of 4–8 solutions.
The plot of electrode potential against the logarithm of current density gives rise to a Tafel plot shown in Figure 1.2. From this plot, a logarithm of corrosion current density can be obtained. The Evans diagrams obtained by the extrapolation of Tafel slopes for the cathodic and anodic polarization curves shown in Figure 1.2 can also been seen in Figures 1.3 and 1.4. In general, the cathodic Tafel slopes are reproducible and reliable for evaluation of corrosion rates as they represent noncorroded original surface of the metal. It is obvious that the corrosion current is greater in acidic solution. The influence of pH also depends on the composition of the alloy as seen in Figure 1.4. When the zinc is present with mercury amalgam, the corrosion current is lower than when the metal is zinc alone. When zinc is present along with platinum, high corrosion rates are observed as platinum provides effective cathodic sites for hydrogen evolution. In addition to this, the stability of the passive film in acid, neutral, or alkaline pH is a contributing factor. Some examples are the stability of magnesium fluoride in alkaline medium and the amphoteric nature of aluminum oxide in pH of 4–8 solutions.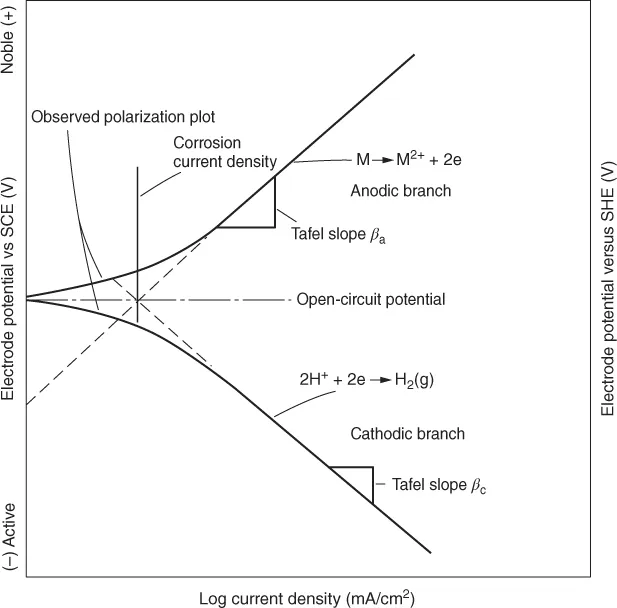 Figure 1.2 Theoretical Tafel plots. (Reproduced by permission, ASM International (4).)
Figure 1.2 Theoretical Tafel plots. (Reproduced by permission, ASM International (4).)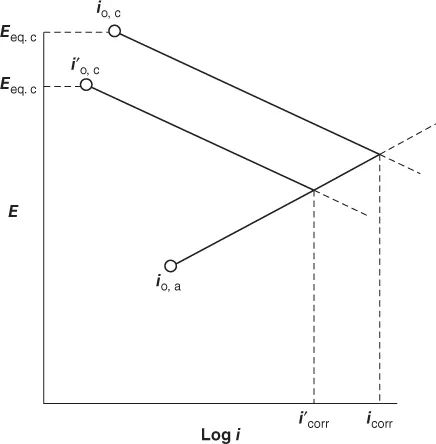 Figure 1.3 Evans diagram for corrosion of zinc as a function of pH. (Reproduced by permission, Elsevier Ltd., (2).)
Figure 1.3 Evans diagram for corrosion of zinc as a function of pH. (Reproduced by permission, Elsevier Ltd., (2).)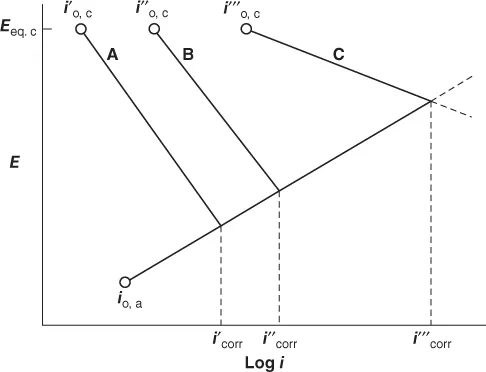 Figure 1.4 Evans diagram for corrosion of zinc alloys. (Reproduced by permission, Elsevier Ltd., (2).)
Figure 1.4 Evans diagram for corrosion of zinc alloys. (Reproduced by permission, Elsevier Ltd., (2).) - The difference in temperature can create a corrosion cell in the case of copper tubes. In general, increase in temperature results in increased corrosion rate. The corrosion rate of steel in acid solutions doubles for an increase of 10 °C between 15 and 70 °C. At temperatures above 70 °C, the solubility of oxygen in aqueous solutions is low, and the rate of reaction cannot be doubled.
- Protective passive films similar to that of stainless steels result in uniform corrosion because of the mobility of the active sites that passivate readily. Corrosion products and/or passive films are characteristic of numerous electrochemical reactions of the alloys. The film is protective depending on coverage capacity, conductivity, partial pressure, porosity, toughness, hardness, and resistance to chemicals and gases. Rust, oxides of iron, and zinc oxide (white rust) are not protective, while patina (CuO), Al2O3, MgO, and Cr2O3 are protective in certain environments. Corrosion is generally controlled by diffusion of active species through the film.
1.2 GALVANIC CORROSION
When a metal or alloy is electrically coupled to another metal or conducting nonmetal in the same electrolyte, a galvanic cell is formed. The electromotive force and the current of the galvanic cell depend on the properties of the electrolyte and the polarization characteristics of the anodic and the cathodic reactions. Galvanic corrosion is caused by the cont...
Table of contents
- Cover
- Title Page
- Copyright
- Table of Contents
- Dedication
- PREFACE
- ACKNOWLEDGMENTS
- CHAPTER 1: INTRODUCTION AND FORMS OF CORROSION
- CHAPTER 2: CORROSION COSTS
- CHAPTER 3: CORROSION CAUSES
- CHAPTER 4: CORROSION CONTROL AND PREVENTION
- CHAPTER 5: CONSEQUENCES OF CORROSION
- INDEX
- End User License Agreement