
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Mammalian Toxicology
About this book
Mammalian Toxicology surveys chemical agents and examines how such chemicals impact on human health, emphasizing the importance in minimizing environmental exposure to chemical and physical hazards in our homes, communities and workplaces through such media as contaminated water, soil and air.
Starting with the basic principles on a wide range of toxic agents, this textbook describes how they enter the body, their mechanisms of action once inside, and strategies for diagnosis, prevention and treatment.
Topics covered include:
- General principles of toxicology: pharmacological and toxicological principles underpinning the study of toxicology, risk assessments and mechanisms of cell death
- Disposition: routes of chemical exposures, entry into the body and various tissues, storage, metabolic biotransformation and elimination, with examples from various toxicants.
- Toxic agents: the occurrences, disposition in the body, health effects, toxic mechanisms, antidotes and treatments of a range of agents including pesticides, metals, solvents, gases, nanomaterials, food components and additives, pharmaceuticals, drugs of abuse, natural toxins, endocrine disruptors, radiation, and warfare weapons.
- Toxic effects: including neurotoxicity, developmental toxicity, immunotoxicity, teratogenecity, male and female reproductive toxicity, mutagenecity, carcinogenicity, pulmonary toxicity, cardiovascular toxicity, hepatotoxicity, gastrointestinal toxicity and cardiovascular toxicity
- Toxicology and society: epidemiological studies of chemical-induced diseases in human populations, and a vision for toxicology in the 21st century.
Mammalian Toxicology is an essential primer for students of toxicology, biochemistry, biology, medicine and chemistry. It is also appropriate for professional toxicologists in research or regulatory affairs, and anyone who needs to understand the adverse effects of toxic agents on the human body.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1
General Principles
1.1 Introduction
1.1.1 Definition of Toxicology
- Chemical and biological toxicology, which is concerned with studies in experimental animals and the environment.
- Clinical toxicology, which is concerned with studies in humans.
- Forensic toxicology, which involves post-mortem studies.
1.1.2 Toxicological Studies
- Toxic agents: source, physical state, chemistry.
- Effects: carcinogenicity, teratogenicity, mutagenicity, neurotoxicology, etc.
- Target organ: nervous system, liver, kidney, reproductive system, blood, etc.
- Testing (Industry)
- Mechanistic studies (Academia)
- Regulatory (Government)
1.1.3 Accreditation in Toxicology
- American Board of Toxicology (ABT, Diplomate), 1980
- Academy of Toxicological Sciences (ATS, Fellow), 1981
1.1.4 Societies of Toxicology
- Society of Toxicology (SOT)
- North Carolina Chapter of Society of Toxicology
- Neurotoxicology Specialty Section of the Society of Toxicology
- American College of Toxicology (ACT)
- Society of Environmental Toxicology and Chemistry (SEMTAC)
- International Neurotoxicology Association (INA)
- American Chemical Society (Agricultural Chemistry)
- American Society of Pharmacology and Experimental Therapeutics (Toxicology Section)
- International Union on Toxicology (IUTOX)
- Society of Neurosciences (Neurotoxicology Section)
- American Society for Investigative Pathology
1.2 Toxic Responses to Xenobiotics
1.2.1 Molecular Changes
- Interactions with nucleic acids (e.g., DNA, RNA), leading to irreversible conformational changes, and causing mutations or carcinogenesis.

- Interactions with proteins, leading to denaturation, precipitation, allosteric effects (change in reactivity), or enzyme inhibition.
1.2.2 Subcellular Changes
- Action on the permeability of cell membranes and disturbances of energy metabolism (ATPase), for example, free radicals.
- Decrease of the stability of lysosomal membranes, resulting in the release of hydrolases and leading to disruption of the cell.
1.2.3 Cellular Changes
1.2.4 Allergic or Sensitization Reactions
- A chemical functions as a hapten:

- Immune cells produce antibodies against the antigen:
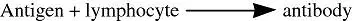
- Subsequent exposure to the chemical yields an antigen, resulting in antigen–antibody interaction. This produces typical manifestations of allergy, such as dermatitis, itching, watery eyes, or bronchiolar constriction.
1.2.5 Idiosyncrasy
Table of contents
- Cover
- Title page
- Copyright
- Dedication
- About the Editor
- List of Contributors
- Acknowledgments
- Introduction
- 1 General Principles
- 2 Alternatives to In-Vivo Studies in Toxicology
- 3 The Application of Omics Technologies to the Study of Mammalian Toxicology
- 4 Cell Death Pathways in Toxicological Response
- 5 Principles of Toxicokinetics and Predictive Toxicokinetics Modeling
- 6 Metabolic Biotransformation of Xenobiotics
- 7 Pesticides
- 8 Metal Toxicology
- 9 Organic Solvents
- 10 Gases
- 11 Nanotoxicology: Environmental, Health and Safety (EHS) Considerations for Assessing Hazards and Risks Following Nanoparticle Exposures
- 12 Pharmaceutical Toxicity In Humans
- 13 Food Additives
- 14 Endocrine Disruptors
- 15 Ionizing Radiation: Toxicologic Action
- 16 Immune System Toxicity and Immunotoxicity Hazard Identification
- 17 Carcinogenicity and Genotoxicity
- 18 Neurotoxicity
- 19 Cardiovascular Toxicology and Its Evaluation
- 20 Liver Toxicology
- 21 Male Reproductive Toxicology: Environmental Exposures versus Reproductive Competence
- 22 Female Reproductive Toxicology
- 23 Pulmonary Toxicology
- 24 Gastrointestinal Toxicology
- 25 Epidemiology
- 26 Drugs of Abuse
- 27 Naturally Occurring Toxins
- 28 Toxicology in the 21st Century
- Index
- EULA
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app