
NMR in Pharmaceutical Science
- English
- ePUB (mobile friendly)
- Available on iOS & Android
NMR in Pharmaceutical Science
About this book
NMR in Pharmaceutical Sciences is intended to be a comprehensive source of information for the many individuals that utilize MR in studies of relevance to the pharmaceutical sector. The book is intended to educate and inform those who develop and apply MR approaches within the wider pharmaceutical environment, emphasizing the toolbox that is available to spectroscopists and radiologists.
This book is structured on the key processes in drug discovery, development and manufacture, but underpinned by an understanding of fundamental NMR principles and the unique contribution that NMR (including MRI) can provide. After an introductory chapter, which constitutes an overview, the content is organised into five sections. The first section is on the basics of NMR theory and relevant experimental methods. The rest follow a sequence based on the chronology of drug discovery and development, firstly 'Idea to Lead' then 'Lead to Drug Candidate', followed by 'Clinical Development', and finally 'Drug Manufacture'. The thirty one chapters cover a vast range of topics from analytical chemistry, including aspects involved in regulatory matters and in the prevention of fraud, to clinical imaging studies.
Whilst this comprehensive volume will be essential reading for many scientists based in pharmaceutical and related industries, it should also be of considerable value to a much wider range of academic scientists whose research is related to the various aspects of pharmaceutical R&D; for them it will supply vital understanding of pharmaceutical industrial concerns and the basis of key decision making processes.
About eMagRes Handbooks
eMagRes (formerly the Encyclopedia of Magnetic Resonance) publishes a wide range of online articles on all aspects of magnetic resonance in physics, chemistry, biology and medicine. The existence of this large number of articles, written by experts in various fields, is enabling the publication of a series of eMagRes Handbooks on specific areas of NMR and MRI. The chapters of each of these handbooks will comprise a carefully chosen selection of eMagRes articles. In consultation with the eMagRes Editorial Board, the eMagRes handbooks are coherently planned in advance by specially-selected Editors, and new articles are written to give appropriate complete coverage. The handbooks are intended to be of value and interest to research students, postdoctoral fellows and other researchers learning about the scientific area in question and undertaking relevant experiments, whether in academia or industry.
Have the content of this handbook and the complete content of eMagRes at your fingertips!
Visit: www.wileyonlinelibrary.com/ref/eMagRes
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Part D
Lead to Drug Candidate
Chapter 14
NMR-based Structure Determination of Drug Leads and Candidates
- 14.1 Introduction
- 14.2 Background to Structure Determination by NMR in the Pharmaceutical Industry
- 14.3 Information from NMR Experiments for Structure Determination
- 14.4 Constitution. Use of C NMR Chemical Shift Predictions and H–C Chemical Shift Correlations from HSQC and HMBC Spectra
- 14.5 Stereochemistry Problems. Proton–Proton Through-space Correlations
- 14.6 Use of the N Isotope
- 14.7 Examples Where H NMR Spectra are Broad or Contain More than One Species in Solution
- 14.8 Conclusions
- References
14.1 Introduction
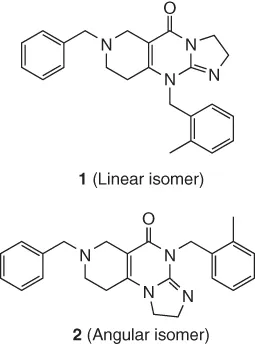
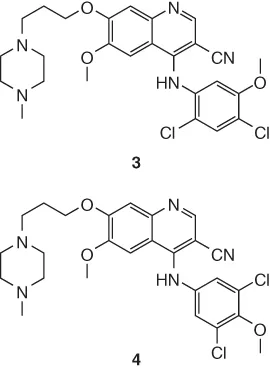
14.2 Background to Structure Determination by NMR in the Pharmaceutical Industry
Table of contents
- Cover
- Series Page
- Title Page
- Copyright
- Table of Contents
- eMagRes
- International Advisory Board
- Contributors
- Series Preface
- Preface
- Abbreviations and Acronyms
- Part A: Introduction
- Part B: NMR Theory & Experimental Methods
- Part C: Idea to Lead
- Part D: Lead to Drug Candidate
- Part E: Clinical Development
- Part F: Drug Manufacture
- Index
- End User License Agreement
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app