
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
Stereoelectronic Effects illustrates the utility of stereoelectronic concepts using structure and reactivity of organic molecules
- An advanced textbook that provides an up-to-date overview of the field, starting from the fundamental principles
- Presents a large selection of modern examples of stereoelectronic effects in organic reactivity
- Shows practical applications of stereoelectronic effects in asymmetric catalysis, photochemical processes, bioorganic chemistry and biochemistry, inorganic and organometallic reactivity, supramolecular chemistry and materials science
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Stereoelectronic Effects by Igor V. Alabugin in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Organic Chemistry. We have over one million books available in our catalogue for you to explore.
Information
1
Introduction
When people thought the earth was flat, they were wrong. When people thought the earth was spherical, they were wrong. But if you think that thinking the earth is spherical is just as wrong as thinking the earth is flat, then your view is wronger than both of them put together. I. Asimov
1.1 Stereoelectronic effects – orbital interactions in control of structure and reactivity
It is easy to believe that the Earth is flat when driving through the Great Plains. Furthermore, the “flat Earth” approximation works quite well in many other aspects of everyday life. Because the small deviation from planarity – only 8 inches per mile – does not make a difference for everyday activities, we can order a cup of coffee or play a game of golf without worrying about the fine details of planetary shapes. However, once one prepares to launch a satellite instead of a golf ball or to navigate “around the globe”, the planet’s curvature becomes crucial. But is Earth a globe? A closer look from space finds that Earth is not a sphere but an “oblate spheroid” that bulges at the equator. Another revision! When should refinements stop and why should a chemist care?
The story of the flat Earth, borrowed from Isaac Asimov,1 reflects the common evolution of scientific models. Sometimes, models are discarded completely (e.g. phlogiston) but, more often, they are refined and taken to the next level of applicability (such as Newton’s theory of gravity paving the way for Einstein’s theory of relativity). How does it apply to organic chemistry? How adequate are the undergraduate organic foundations for the broad understanding of structure and reactivity? Do we really need to go deeper?
The importance of continuous improvement of models is illustrated by the following “diagnostic quiz” given to first-year graduate students at the Florida State University. Take a minute and test yourself.
The answers may or may not be surprising, depending on how far the reader is separated from the undergraduate organic class. For each pair in Figure 1.1, the bottom structure is more stable than the top structure. In particular, the gauche conformation of 1,2-difluoroethane is more stable than the anti conformations; cis-difluoroethene is more stable than the trans-isomer; the equatorial conformers of the two fluoro-substituted oxacyclohexanes are less stable than their axial counterparts; and the diaxial 1,4-difluorocyclohexane is ~1 kcal/mol more stable than the diequatorial conformer. The answer in each case is opposite to expectations based on the steric repulsion – the “flat Earth” models that have served reasonably well as a foundation of undergraduate organic chemistry.
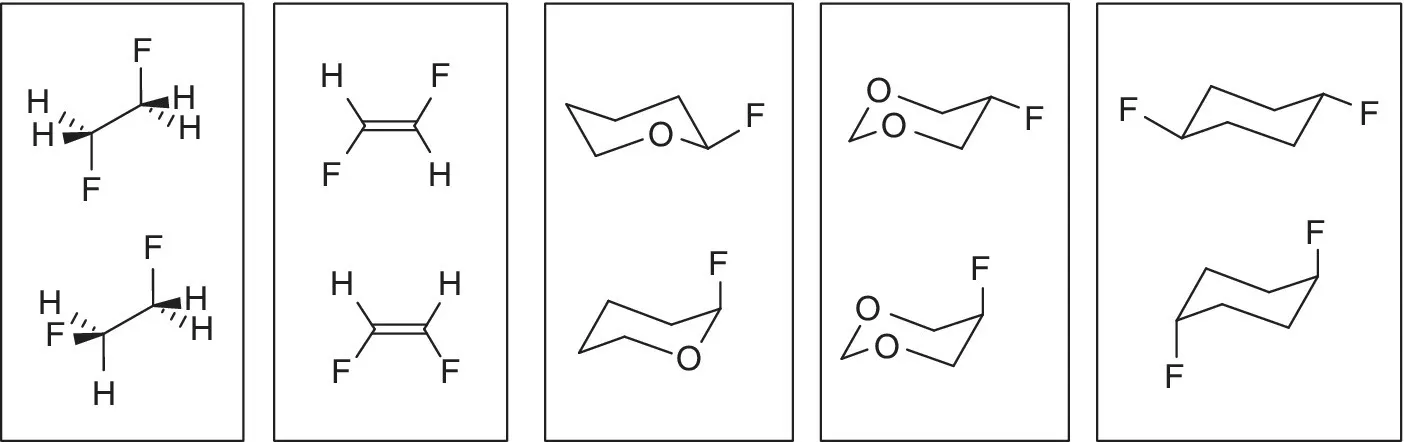
Figure 1.1 Circle the more stable structure in each of the above pairs.
It is not surprising that it is a rare undergraduate student who gives correct answers to all of the above problems. Almost invariably, the correct answers come as a surprise, even to a student with a good mastery of undergraduate organic chemistry. Clearly, a new set of concepts is needed to refine the initial model of organic structure and reactivity. This book aims to introduce these concepts in a way that will provide a logical ascension from a simplified discussion of an undergraduate textbook to a level appropriate for a practicing organic chemist.
Undergraduate organic chemistry lays the foundation of chemical knowledge – a reasonable approximation and a useful and often sufficient way to describe molecules as Lewis structures augmented, as needed, by resonance. However, once one realizes that organic molecules are quantum objects delocalized in space, far from the flat two-dimensional drawings on a sheet of paper or a blackboard, it may not be a complete surprise that this simple concept has its limitations.
The way to get to the next step in understanding molecular structure is to move from the flat Lewis structures on a flat sheet of paper to the 3rd dimension. The elements of stereochemistry are introduced, of course, in undergraduate courses. However, this important step is not enough – when one needs to design, understand, and control new reactions, it is crucial to start thinking about organic molecules as intrinsically delocalized and spatially anisotropic quantum objects. This book focuses on the importance of delocalization – the deviation of real molecules, quantum objects par excellence, from idealized Lewis structures.
The laws of chemical attraction in the world of atoms and molecules are defined by quantum mechanics. Constructive interference of electronic wavefunctions is the quantum essence of chemical bonding that “glues” smaller fragments into larger molecular assemblies. As a result, the chemical world at the molecular level is defined by interactions between atomic and molecular orbitals. Because orbitals and molecules are three-dimensional, such interactions depend on the relative atomic arrangements in space. The modulations of electronic interactions by changes in molecular geometry are generally referred to as stereoelectronic effects. In organic chemistry, stereoelectronic effects can be defined as stabilizing electronic interactions maximized by a particular geometric arrangement which can be traced to a favorable orbital overlap. Stereoelectronic interactions are omnipresent in chemistry, as only a small subgroup of electronic effects, i.e. the long-range2 electrostatic effects, can be considered, with a degree of approximation, as not having a substantial stereoelectronic component.
There is one common misunderstanding that needs to be addressed early: “stereoelectronic” is not the same as “steric + electronic”! By definition, stereoelectronic effects are always stabilizing, reflecting increased delocalization at favorable conformations. Repulsive steric interactions also depend on the arrangement of orbitals in space but, historically, are not included under the umbrella of stereoelectronic effects.
Stereoelectronic factors control interactions between different atoms or molecules and interactions between different parts of a single molecule. Although our focus...
Table of contents
- Cover
- Title Page
- Table of Contents
- Acknowledgement
- Supplementary Material
- 1 Introduction
- 2 Direct Effects of Orbital Overlap on Reactivity
- 3 Beyond Orbital Overlap
- 4 Computational and Theoretical Approaches for Studies of Stereoelectronic Effects
- 5 General Stereoelectronic Trends – Donors, Acceptors, and Chameleons
- 6 Stereoelectronic Effects with Donor and Acceptor Separated by a Single Bond Bridge
- 7 Stereoelectronic Effects with Donor and Acceptor Separated by a Vinyl Bridge
- 8 Remote Stereoelectronic Effects
- 9 Transition State Stabilization with Stereoelectronic Effects
- 10 Stereoelectronic Effects in Reaction Design
- 11 Stereoelectronic Effects in Action
- 12 Probing Stereoelectronic Effects with Spectroscopic Methods
- Index
- End User License Agreement