
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Essentials of Inorganic Materials Synthesis
About this book
This compact handbook describes all the important methods of synthesis employed today for synthesizing inorganic materials. Some features:
- Focuses on modern inorganic materials with applications in nanotechnology, energy materials, and sustainability
- Synthesis is a crucial component of materials science and technology; this book provides a simple introduction as well as an updated description of methods
- Written in a very simple style, providing references to the literatureto get details of the methods of preparation when required
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1
INTRODUCTION
Much chemical ingenuity is involved in the synthesis of solid materials [1–6] and this aspect of material science is getting increasingly recognized as a crucial component of the subject. Tailor-making materials of the desired structure and properties is the main goal of material science and solid-state chemistry, but it may not always be possible to do so. While one can evolve a rational approach to the synthesis of solid materials [7], there is always an element of serendipity, encountered not so uncommonly. A good example of an oxide discovered in this manner is NaMo4O6 (Fig. 1.1) containing condensed Mo6 octahedral metal clusters [8]. This was discovered by Torardi and McCarley in their effort to prepare the lithium analogue of NaZn2Mo3O8. Another chance discovery is that of the phosphorus–tungsten bronze, RbxP9W32O112, formed by the reaction of phosphorus present in the silica of the ampoule, during the preparation of the Rb–WO3 bronze [9]. Since the material could not be prepared in a platinum crucible, it was suspected that a constituent of the silica ampoule must have got incorporated. This discovery led to the synthesis of the family of phosphorus–tungsten bronzes of the type AxP4O8 (WO3)2m. Chevrel compounds of the type AxMO6S8 (A = Cu, Pb, La etc.) shown in Figure 1.2 were also discovered accidentally [10].
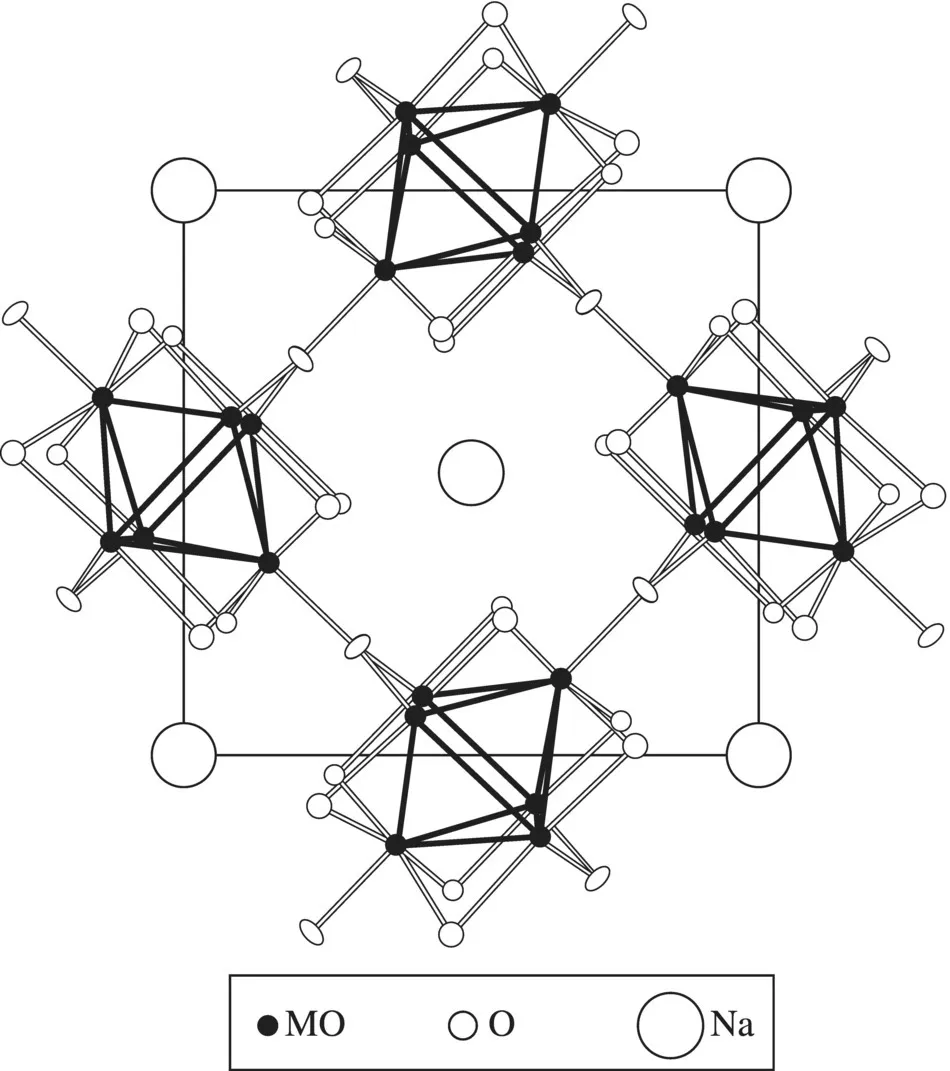
Figure 1.1 Structure of NaMo4O6.
(From Ref. 8, Torardi et al., J. Am. Chem. Soc., 101 (1979) 3963. © 1979, American Chemical Society)
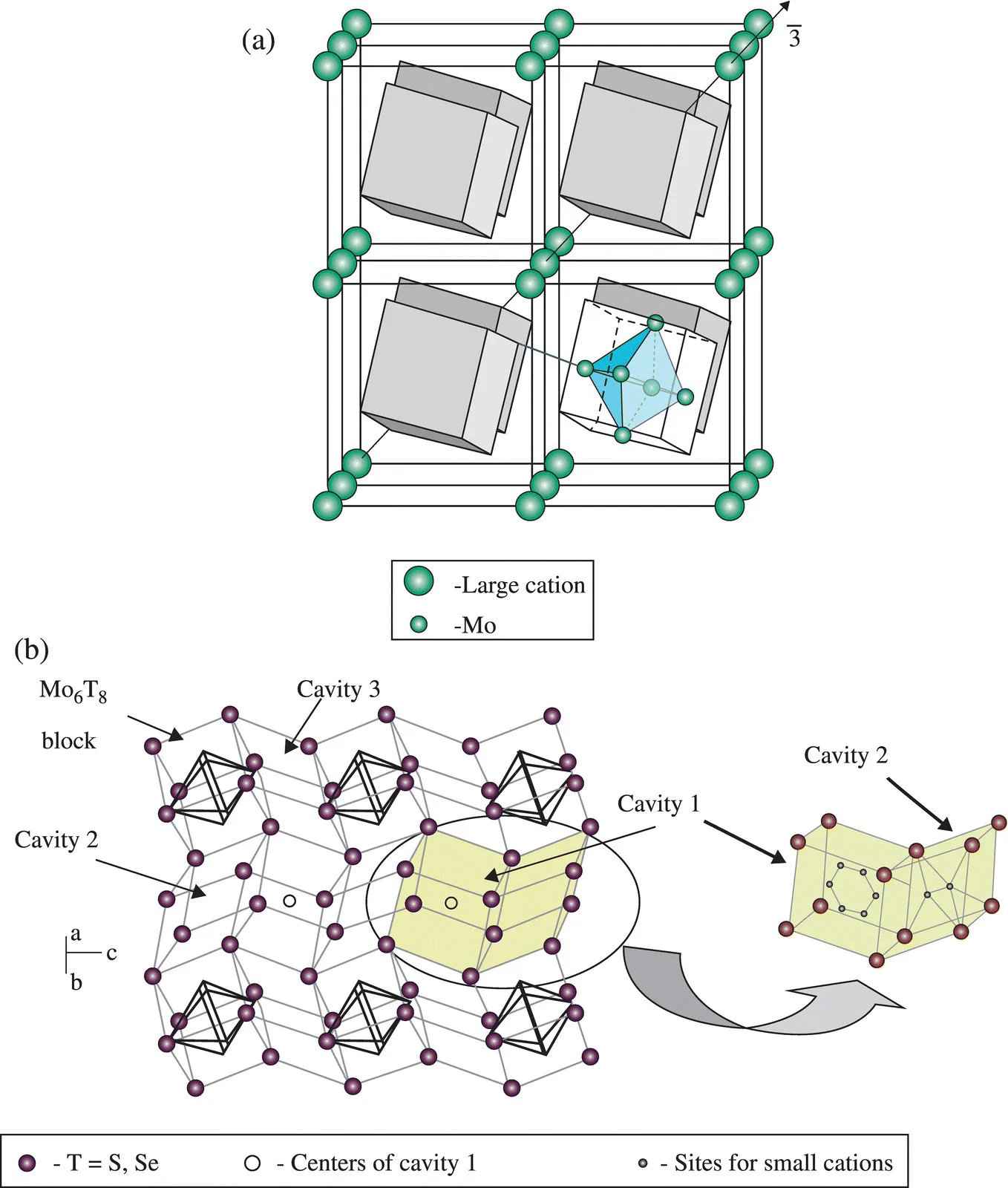
Figure 1.2 Crystal structure of Chevrel phases. (a) Type I with large cation in the origin (eight rhombohedral unit cells): each cation is surrounded by eight Mo6T8 blocks. The internal structure is shown for one of the blocks. Intercluster Mo–T1 bond is marked in blue. (b) Three types of pseudocubic cavities between the Mo6T8 blocks. Cavities 1 and 2 form the diffusion channels in three directions (a channel in one of the directions is shown here). Sites for small cations in cavities 1 and 2 are presented separately on the right.
Rational synthesis of materials requires knowledge of crystal chemistry besides thermodynamics, phase equilibria and reaction kinetics. There are several examples of rational synthesis. A good example is SIALON [11], where Al and oxygen were partly substituted for Si and nitrogen in Si3N4. The fast Na+ ion conductor NASICON, Na3Zr2PSi2O12 (Fig. 1.3), was synthesized with a clear understanding of the coordination preferences of the cations and the nature of the oxide networks formed by them [12]. The zero-expansion ceramic Ca0.5Ti2P3O12 possessing the NASICON framework was later synthesized based on the idea that the property of zero-expansion would be exhibited by two or three coordination polyhedra linked in such a manner as to leave substantial empty space in the network [7]. Synthesis of silicate-based porous materials, making use of organic templates to predetermine the pore or cage geometries, is well known [13]. A microporous phosphate of the formula (Me4N)1.3(H3O)0.7 Mo4O8(PO4)2⋅2H2O, where the tretramethyl–ammonium ions fill the voids in the 3-dimensional structure made up of Mo4O8 cubes and PO4 tetrahedra, has been prepared in this manner [14].
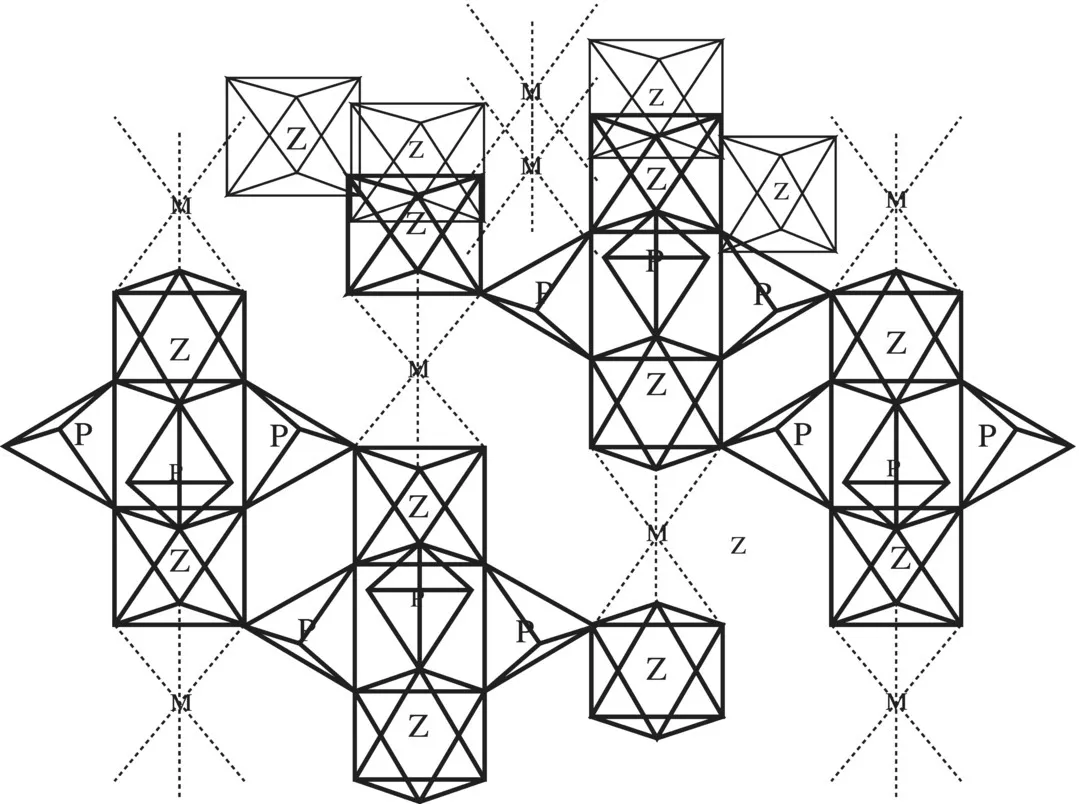
Figure 1.3 Structure of NaZr2(PO4)3 which provided the design for NASICON: vacant trigonal–prismatic sites, p; octahedral Zr4+ sites, Z; and octahedral sites available for Na+, M. For each M, there are three Mo sites forming hcp layers perpendicular to the c-axis.
A variety of inorganic solids have been prepared in the past several years by the traditional ceramic method, which involves mixing and grinding powders of the constituent oxides, carbonates and such compounds, and heating them at high temperatures with intermediate grinding when necessary. A wide range of conditions, often bordering on the extreme, such as high temperatures and pressures, very low oxygen fugacities and rapid quenching, have been employed in material synthesis. Low-temperature chemical routes and methods involving mild reaction conditions are, however, of greater interest. The present-day trend is to avoid brute-force methods in order to get a better control of the structure, stoichiometry and phasic purity. Soft-chemistry routes, which the French call chimie douce, are indeed desirable because they lead to novel products, many of which are metastable and cannot otherwise be prepared. Soft-chemistry routes essentially make use of simple reactions such as intercalation, ion exchange, hydrolysis, dehydration and reduction that can be carried out at relatively low temperatures. The topochemical nature of certain solid-state reactions is also exploited in synthesis. Ion exchange, intercalation and many other types of reactions are generally topochemical.
Many of the materials that are prepared are metastable. Metastable phases possess higher free energy than the corresponding stable phases of the same composition. Metastability can arise from frozen disorder and/or defects (e.g. glasses, ionic conductors). Topological metastability is found in porous materials including zeolites. Nanocrystals of many materials crystallize in metastable str...
Table of contents
- COVER
- TITLE PAGE
- TABLE OF CONTENTS
- AUTHOR BIOGRAPHIES
- PREFACE
- 1 INTRODUCTION
- 2 COMMON REACTIONS EMPLOYED IN SYNTHESIS
- 3 CERAMIC METHODS
- 4 DECOMPOSITION OF PRECURSOR COMPOUNDS
- 5 COMBUSTION SYNTHESIS
- 6 ARC AND SKULL METHODS
- 7 REACTIONS AT HIGH PRESSURES
- 8 MECHANOCHEMICAL AND SONOCHEMICAL METHODS
- 9 USE OF MICROWAVES
- 10 SOFT CHEMISTRY ROUTES
- 11 NEBULIZED SPRAY PYROLYSIS
- 12 CHEMICAL VAPOUR DEPOSITION AND ATOMIC LAYER DEPOSITION
- 13 NANOMATERIALS
- 14 MATERIALS
- INDEX
- END USER LICENSE AGREEMENT
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Essentials of Inorganic Materials Synthesis by C. N. R. Rao,Kanishka Biswas in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Energy. We have over one million books available in our catalogue for you to explore.