
Postgraduate Haematology
- English
- ePUB (mobile friendly)
- Available on iOS & Android
About this book
This comprehensive textbook is the key resource for postgraduate trainees or residents in haematology. Now in its seventh edition, the book continues to provide everything the reader needs for examination preparation or clinical practice.
Postgraduate Haematology discusses up-to-date knowledge of the pathogenesis, clinical and laboratory features, management and treatment of a wide range of blood and bone marrow disorders in a concise and user friendly style. It presents essential information for everyday use and teaching, as well more detailed scientific background for more in-depth reading, accompanied by thoughtful referencing.
The clearly illustrated full-colour figures and charts demonstrate key facts, and are supplemented by numerous high quality photomicrographs of blood cells and tissues. Over 51 chapters from international authors, including the WHO Classification of Haematopoietic and Lymphoid Tissues, Postgraduate Haematology provides an expert review of malignant and non-malignant haematology.
- New sections reflect advances in the specialty, e.g. knowledge gained from new generation sequencing, latest anticoagulant drugs, diagnostic laboratory tools, and treatment strategies
- Superb four-color illustrations and photomicrographs of blood cells and tissues throughout
- Includes algorithms to aid with decision-making for treatment
- Companion website featuring figures and tables from the book
Arm yourself with the textbook of choice for trainees and practitioners in haematology.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
CHAPTER 1
Stem cells and haemopoiesis
Emma de Pater and Elaine DzierzakErasmus Stem Cell Institute, Erasmus Medical Centre, Rotterdam, Netherlands and University of Edinburgh, Centre for Inflammation Research, UK
Introduction
Hierarchical organization and lineage relationships in the adult haemopoietic system
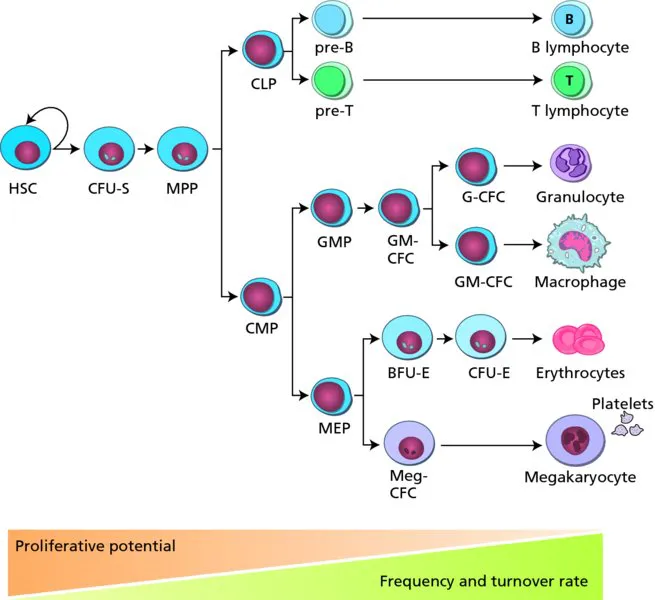
Sites of adult haemopoiesis
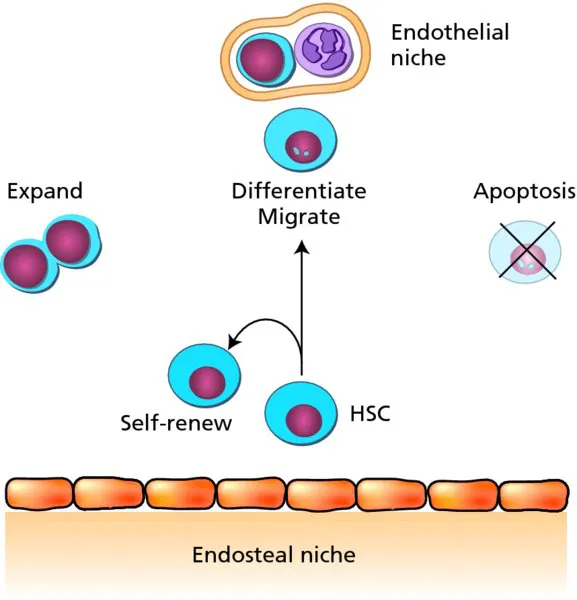
Development of HSCs
Waves of haemopoietic generation in embryonic development
Table of contents
- Cover
- Companion website
- Title page
- Copyright
- Contributor list
- Preface to the seventh edition
- Preface to the first edition
- Chapter 1 Stem cells and haemopoiesis
- Chapter 2 Erythropoiesis
- Chapter 3 Iron metabolism, iron deficiency and disorders of haem synthesis
- Chapter 4 Iron overload
- Chapter 5 Megaloblastic anaemia
- Chapter 6 Haemoglobin and the inherited disorders of globin synthesis
- Chapter 7 Sickle cell disease
- Chapter 8 Hereditary disorders of the red cell membrane and disorders of red cell metabolism
- Chapter 9 Acquired haemolytic anaemias
- Chapter 10 Inherited aplastic anaemia/bone marrow failure syndromes
- Chapter 11 Acquired aplastic anaemia and paroxysmal nocturnal haemoglobinuria
- Chapter 12 Red cell immunohaematology
- Chapter 13 Clinical blood transfusion
- Chapter 14 Phagocytes
- Chapter 15 Lysosomal storage disorders
- Chapter 16 Normal lymphocytes and non-neoplastic lymphocyte disorders
- Chapter 17 The spleen
- Chapter 18 The molecular basis of haematological malignancies
- Chapter 19 Laboratory diagnosis of haematological neoplasms
- Chapter 20 Acute myeloid leukaemia
- Chapter 21 Adult acute lymphoblastic leukaemia
- Chapter 22 Childhood acute lymphoblastic leukaemia
- Chapter 23 Supportive care in the management of leukaemia
- Chapter 24 Chronic myeloid leukaemia
- Chapter 25 The myelodysplastic syndromes
- Chapter 26 Myeloproliferative neoplasms
- Chapter 27 Chronic lymphocytic leukaemia and other chronic B-cell disorders
- Chapter 28 T-cell lymphoproliferative disorders
- Chapter 29 Multiple myeloma
- Chapter 30 Amyloidosis
- Chapter 31 The classification of lymphomas: updating the WHO classification
- Chapter 32 Hodgkin lymphoma
- Chapter 33 Non-Hodgkin lymphoma: low grade
- Chapter 34 Non-Hodgkin lymphoma: high grade
- Chapter 35 Stem cell transplantation
- Chapter 36 Normal haemostasis
- Chapter 37 The vascular function of platelets
- Chapter 38 Haemophilia and Von Willebrand Disease
- Chapter 39 Rare inherited coagulation disorders
- Chapter 40 Acquired coagulation disorders
- Chapter 41 Congenital platelet disorders
- Chapter 42 Primary immune thrombocytopenia
- Chapter 43 Thrombotic thrombocytopenic purpura and haemolytic–uraemic syndrome (congenital and acquired)
- Chapter 44 Heritable thrombophilia
- Chapter 45 Acquired venous thrombosis
- Chapter 46 Antithrombotic agents
- Chapter 47 Management of venous thromboembolism
- Chapter 48 Haematological aspects of systemic disease
- Chapter 49 Haematological aspects of tropical diseases
- Chapter 50 Neonatal haematology
- Chapter 51 WHO Classification: Tumours of the Haematopoietic and Lymphoid Tissues (2008)
- Index
- EULA