
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
Immunohistochemistry and immunocytochemistry are invaluable tools for the visualization of tissue and cellular antigens in diagnostic and biological research environments. The need to obtain accurate, reliable and reproducible results is paramount.
It is with this fundamental aim in mind that we have compiled Immunohistochemistry: Essential Methods. We have achieved this by examining each aspect of immunochemistry in turn, with each chapter including detailed information regarding the subject matter in question. Each chapter is written by an expert in their field and includes protocols that are typically used in their own research. Subjects covered are, amongst others, antibodies and their production; selection of reporter labels; immunochemical staining methods and experimental design (both using single and multiple reporter labels); quality assurance; automated immunochemistry; confocal microscopy and electron microscopy. In addition, benefits and limitations of each approach are discussed within the chapters.
It is with this fundamental aim in mind that we have compiled Immunohistochemistry: Essential Methods. We have achieved this by examining each aspect of immunochemistry in turn, with each chapter including detailed information regarding the subject matter in question. Each chapter is written by an expert in their field and includes protocols that are typically used in their own research. Subjects covered are, amongst others, antibodies and their production; selection of reporter labels; immunochemical staining methods and experimental design (both using single and multiple reporter labels); quality assurance; automated immunochemistry; confocal microscopy and electron microscopy. In addition, benefits and limitations of each approach are discussed within the chapters.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Immunohistochemistry and Immunocytochemistry by Simon Renshaw in PDF and/or ePUB format, as well as other popular books in Biological Sciences & Molecular Biology. We have over one million books available in our catalogue for you to explore.
Information
Chapter 1
Antibodies for Immunochemistry
Mark Cooper and Sheriden Lummas
Abcam plc, Cambridge, UK
INTRODUCTION
Unlike innate immunity, the adaptive immune response recognizes, reacts to and remembers foreign substances invading an organism. Antibodies play a central role in the function of adaptive immunity. Their roles are to detect, specifically bind and facilitate the removal of foreign substances from the body. Memory B cells create an immunological memory that allows the immune system to respond quicker upon subsequent exposure to the same foreign substance.
A substance not recognized by the immune system as being native to the host and therefore stimulates an immune response is known as an antigen (antibody generator). Binding of an antigen to an antibody is specific. Biochemical research utilizes the ability of antibodies to distinguish between antigens and to detect biological molecules (commonly proteins) in cells and tissues using immunochemical staining techniques. Immunochemistry is the focus of this text, and its practice is discussed in detail throughout later chapters (see p 35).
Typical Antibody Structure
Antibodies are immunoglobulin (Ig) proteins produced by B cells in the presence of an antigen. Immunoglobulins exist as five main classes or isotypes: IgA, IgD, IgE, IgG and IgM. Each isotype performs a different function in the immune system. IgG has a long half-life in serum (Table 1.1), which means its clearance from the circulatory system is slow. The abundance and retention of IgG in circulation compared to the other classes make it the most common antibody isotype reagent used in biochemical research.
Table 1.1 A Comparison of Immunoglobulin ClassesIgG has the longest half-life of all the antibody classes and is produced during the secondary immune response. IgG, IgD and IgE are monomeric structures consisting of a single antibody unit. IgA can occur as a monomer or dimer (two units). IgM exists as a pentameric molecule, with five basic immunoglobulin units joined by an additional polypeptide chain (J chain), making it the largest antibody class with a molecular weight of 970 kDa
| Immunoglobulin | |||||||||
| IgG1 | IgG2 | IgG3 | IgG4 | IgM | IgA1 | IgA2 | IgD | IgE | |
| Molecular weight (kDa) | 146 | 146 | 165 | 146 | 970 | 160 | 160 | 184 | 188 |
| Serum level (mean adult mg ml−1) | 9 | 3 | 1 | 0.5 | 1.5 | 3 | 0.5 | 0.03 | 5 × 10−5 |
| Half-life in serum (days) | 21 | 20 | 7 | 21 | 10 | 6 | 6 | 3 | 2 |
| Location | Bloodstream. Can pass through blood vessel walls readily and cross into the placenta. | Bloodstream | Body secretions: tears, sweat, saliva; breast milk | B-cell surface | Bound to mast cells | ||||
| Function | Activates complement pathway | Produced during primary immune response and activates complement system | Form a defence on the surface of body cells. Immune protection to newborn. | Unknown | Stimulates allergy response | ||||
The basic antibody unit is shared across all five isotypes. Two identical heavy (H) and light (L) polypeptide chains connected by a disulfide bond form the commonly illustrated Y-shaped antibody structure (Fig. 1.1). The arms of the Y structure form the Fab (fragment antigen-binding) region while the base is the Fc (fragment crystallizable) region.
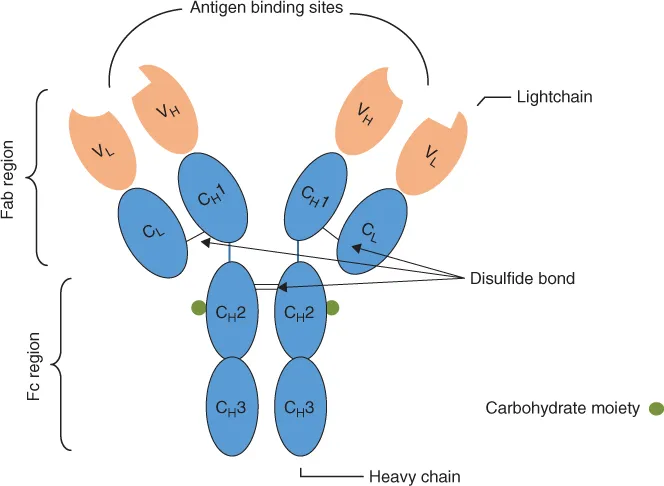
Figure 1.1 A Schematic Diagram of an Immunoglobulin MoleculeThe basic antibody molecule is a Y-shaped structure consisting of two heavy and two light polypeptide chains joined by disulfide bonds. The chains are composed of variable (orange) and constant (blue) immunoglobulin domains. The antibody–antigen binding site (paratope) is located at the tip of the Y arms. The Fab and Fc regions of the molecule are tethered together by a hinge region, which provides the antibody molecule with flexibility. Constant domains share the same amino acid sequence for a given antibody isotype. Different immunoglobulin isotypes arise from subtle sequence variations in the constant domains.
Both H and L chains consist of variable (V) and constant (C) domains, named according to the conservation of their amino acid sequence. One variable domain is present for each H chain and L chain and is situated at the amino terminus. VL and VH domains are paired together to create the antigen-binding site (paratope). Specificity of antigen binding is determined by the variation in amino acid sequence in this region. This enables antibodies to recognize a diverse range of antigens, even though only a single amino acid difference between antigens exists. The remainder of the H and L chains are composed of constant domains: one domain in the L chain and three domains in the H chains denoted as CH1, CH2 and CH3. For a given isotype, the entire CH amino acid sequence is conserved. Subtle differences in the sequence occur and give rise to isotype subclasses (as provided in Table 1.1). IgG sub-classes are present across species, with IgG1, IgG2, IgG2a and IgG3 subclasses existing within mice. The subclasses have different binding affinities for the purification of protein resins, which are discussed in the ‘Antibody Labelling’ Section. It is therefore important for the sub-class to be accurately determined in order to efficiently purify antibodies from sera. Furthermore, primary antibody subclass determination is one of the critical parameters (among others) that enables the end user to select an appropriate secondary antibody.
Differences in CL sequence equate to the type of L chain, out of which two types are found in antibodies. L chains exist as lambda (λ) or kappa (k) and are identically present in one form or the other in a single antibody. At the centre of the Y-shaped structure is the hinge region that acts as a tether, linking the Fab and Fc regions of the molecule. The two Y arms of the Fab region are able to move independently, providing the molecule with flexibility when the antibody binds two identical antigens, particularly when the antigens are distances apart [1]. This is a property that contributes to the use of antibodies in immunoassays. Being glycoproteins, antibodies contain a sugar side chain (carbohydrate moiety). This is bound to the CH2 region and contributes to antibody destination within tissues and the type of immune response initiated depending on antibody class [1].
Antibody Structure Is Optimized for Its Function
In order to understand how antibodies are engineered for their function, an overview of protein structure organization has been presented.
Proteins are composed of polypeptide chains consisting of basic units called amino acids. The consecutive sequence of amino acids is the primary structure. Hydrogen bonds within the polypeptide chain generate alpha helices and beta sheets to create the secondary protein structure. As the chains are pulled into close proximity of each other, additional bonds and interactions form between amino acid side chains, and hydrophobic bonds and van der Waals interactions form between non-polar amino acids. Further reinforcement of the conformational structure is achieved by the formation of disulfide bonds between cysteine sulfhydryl groups. The result of these bonds is the generation of polypeptide subunits, that is, the tertiary protein structure. The arrangement into multi-subunit structures creates the final quaternary protein structure [2]. Two main types of quaternary protein structure exist: globular and fibrous.
Immunoglobulins are a superfamily of globular proteins with roles associated with the immune system. Examples include cell surface receptors (Fc) and antibodies. Members of this superfamily exhibit a common structural motif, that is, the immunoglobulin domain [2]. The immunoglobulin domain is approximately 110 amino acids in length. Two immunoglobulin domains are present in the light chain, whereas the heavy chain has four domains, numbered from the amino (N-) terminus to carboxyl (C-) terminus. Each domain is a sandwich-like structure formed from anti-parallel beta-pleated sheets of the polypeptide chain bound together by disulfide bonds. This structure is known as the immunoglobulin fold. Loops are created at the ends of the immunoglobulin folds where the beta-pleated sheets change the direction. These loops are 5–10 amino acids residues in length and reside within the variable regions, protruding from the surface. They are designated hypervariable (HV) loops because the amino acid sequence variation within this region is considerable. HV loops are also referred to as complementarity-determining regions (CDRs). The three HV loops present in the variable region are denoted HV1, HV2 and HV3, with HV3 containing the greatest sequence variation. The remainder of the variable region is composed of framework regions F...
Table of contents
- Cover
- Title Page
- Copyright
- Table of Contents
- List of Contributors
- Preface
- Acknowledgements
- Chapter 1: Antibodies for Immunochemistry
- Chapter 2: The Selection of Reporter Labels
- Chapter 3: Immunohistochemistry and Immunocytochemistry
- Chapter 4: Multiple Immunochemical Staining Techniques
- Chapter 5: Quality Assurance in Immunochemistry
- Chapter 6: Automated Immunochemistry
- Chapter 7: Confocal Microscopy
- Chapter 8: Ultrastructural Immunochemistry
- Index
- End User License Agreement